Evaluation of chronic prostatitis/chronic pelvic pain syndrome
J. Curtis Nickel 2
1 Department of Urology, Ludwig-Maximilians-University of Munich, Munich, Germany
2 Department of Urology, Queen's University, Kingston, Canada
Abstract
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a common condition defined as chronic (at least 3 months) pain or discomfort in the pelvic region, variably associated with lower urinary tract symptoms, sexual dysfunction and psychosocial consequences with considerable impact on quality of life. Since the exact pathophysiology underlying CP/CPPS is still unknown, no specific test for a definitive diagnosis is available. The main objectives for evaluation are to exclude differential diagnoses associated with pelvic pain and to identify the individual clinical profile or “picture” relevant for a tailored treatment concept. Keystones of basic evaluation include a thorough patient history and comprehensive assessment of symptoms based on subjective outcome measures, like the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI), and clinical phenotyping classification systems, such as UPOINTs. Further diagnostic procedures are adapted to the patient. An effective personalized multi-modal treatment concept relies on this accurate basic evaluation and regular follow-ups to monitor treatment response with the option for modification.
Summary of Recommendations
- Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is defined as pain or discomfort in the pelvic region, variably associated with lower urinary tract symptoms (LUTS), sexual dysfunction and psychosocial consequences, lasting for at least three of the past six months.
- The main objectives for evaluation are to exclude differential diagnoses associated with pelvic pain and to identify the individual clinical profile relevant for a tailored treatment concept.
- Validated symptom-scoring instruments like the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) are recommended for basic evaluation and follow-up (level of evidence 2b, grade of recommendation B). According to associated symptoms additional objective outcome measures are available like the International Prostate Symptom Score (IPSS) for LUTS or the International Index of Erectile Function (IIEF-5) for erectile dysfunction.
- Clinical phenotyping of CP/CPPS patients based on UPOINTs reveals the unique clinical profile or “picture” of individual patients, provides guidance for further diagnostic steps and suggests targets for a phenotypically directed multimodal treatment concept.
- The 4-glass test or the 2-glass test represent the standard method to exclude or confirm chronic bacterial prostatitis (NIH category II), and detect inflammatory components, which are relevant for the classification of CP/CPPS into an inflammatory (NIH category IIIA) or noninflammatory type (NIH category IIIB) (grade of recommendation A).
- Additional diagnostic procedures should be adapted to the patient to exclude other causes of pelvic pain or to assess associated symptoms (grade of recommendation A).
1 Introduction
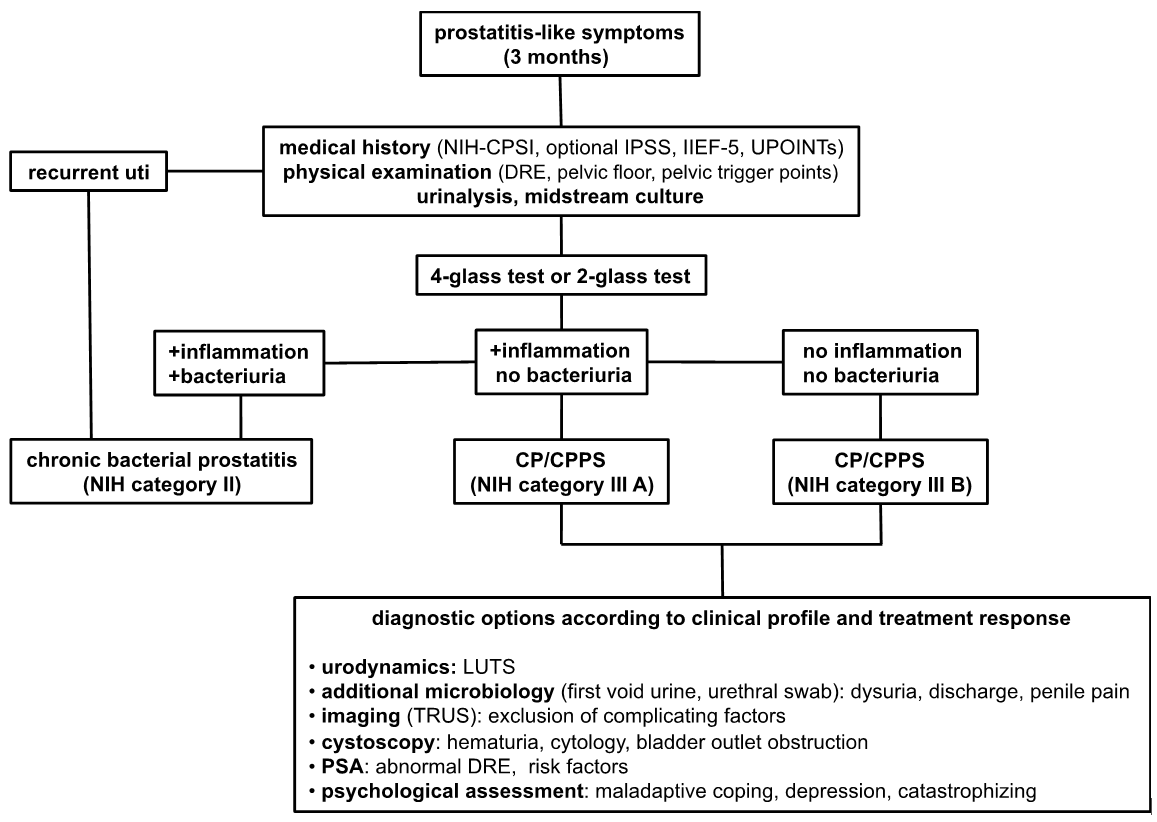
In 1999, the National Institutes of Health established a consensus definition and classification systems subdividing the complex of prostatitis into four categories [1]. Accordingly, prostatitis syndromes comprise acute bacterial prostatitis (NIH category I), chronic bacterial prostatitis (NIH category II), chronic prostatitis/chronic pelvic pain syndrome (NIH category III) and asymptomatic prostatitis (NIH category IV). Per definition, in CP/CPPS no causative bacterial pathogen is detected. Only 5 to 10% of patients with prostatitis symptoms are of bacterial origin [2], [3]. CP/CPPS is further sub-classified into an inflammatory type (NIH category IIIA) and a noninflammatory type (NIH category IIIB) according to the presence of leukocytes in prostatic specimen. Correct classification of patients diagnosed with prostatitis is crucial and requires a systematic diagnostic assessment.
CP/CPPS is defined as pain or discomfort in the pelvic region, many times associated with urinary symptoms, sexual dysfunction and psychosocial consequences, lasting for at least three of the preceding six months. Differential diagnoses such as urinary tract infection, malignancies, anatomic abnormalities or neurologic disorders need to be excluded.
The exact pathophysiology underlying CP/CPPS still remains elusive. As a consequence, no specific biomarkers are available for diagnostic assessment. Further, no efficient mono-therapy has been identified for the treatment of this bothersome condition [4], [5]. This has made the management of CP/CPPS very challenging. With the introduction of an accepted classification system and the development of validated symptom-scoring questionnaires, a focused approach for diagnostic assessment and monitoring of treatment response is possible. The main objective for evaluation of patients suffering from CP/CPPS is to exclude differential diagnoses associated with pelvic pain and to identify the clinical picture relevant for a tailored treatment concept in every individual patient. Reliable and valid outcome measures are useful to evaluate clinical response during follow-up. The present article outlines our current understanding of basic evaluation of patients diagnosed with CP/CPPS.
2 Methods
A systematic literature search was performed in the Pubmed and Cochrane database. We searched for randomized controlled trials, clinical trials, meta-analysis, medical guidelines and systematic reviews published in English language from January 1999 to December 2015. A search using different algorithms including the following MeSH terms was conducted: chronic prostatitis, chronic pelvic pain syndrome, prostate pain syndrome, pelvic pain, evaluation, management, diagnosis. No rating or grading of the selected literature was conducted. Recommendations cited in the manuscript are derived from current guidelines.
3 Results
3.1 Clinical presentation
The main symptom in CP/CPPS is pain perceived in the region of the prostate and/or perineum [6], [7]. On digital rectal examination, patients report moderate tenderness of the prostate to palpation. Radiation of pain to the testes or penis is frequent. Additional pain localizations include rectum, lower abdomen and lower back. An analysis of 1563 patients with CP/CPPS revealed that pain during ejaculation and micturition was present in 45% and 43%, respectively [8]. Of note, ejaculatory pain or discomfort was shown to be a relevant discriminatory item and strong predictor of symptom severity and quality of life [9]. Muscle tenderness and possible musculoskeletal dysfunction of the abdominal and pelvic region are further pain symptoms present in CP/CPPS. Symptoms may remain stable or improve slightly over time, but fluctuations with considerable flare ups can also be observed [10]. About 50 to 60% of patients diagnosed with CP/CPPS report lower urinary tract symptoms (LUTS) [11], [12], [13]. Furthermore, 40 to 70% of patients suffer from sexual dysfunction including erectile and ejaculatory dysfunction and impaired libido [14], [15], [16], [17], [18]. Correlation studies demonstrated the substantial impact of sexual dysfunction on symptoms severity and quality of life [15], [16], [18], [19], [20]. CP/CPPS can also be associated with psychosocial disorders such as anxiety, depression or pain catastrophizing [13], [21], [22], [23]. In particular, pain catastrophizing seems to be a prominent biopsychosocial predictor of poor mental and physical health in CP/CPPS [24]. Since cognitive and behavioral factors appear to impact significantly patient adjustment, these variables should be part of first assessment. Somatic syndromes such as irritable bowel syndrome, fibromyalgia or chronic fatigue syndrome are comorbidities associated with CP/CPPS [25]. For example, 22 to 31,2% of CP/CPPS patients were also diagnosed with irritable bowel disease [26], [27], [28], [29]. So, due to a possible overlap with non-urological conditions an extended evaluation of the clinical manifestations of these more systemic chronic pain conditions needs to be undertaken. Per definition, the individual spectrum of symptoms is experienced for at least 3 months of the preceding 6 months. This brief outline on clinical manifestations in CP/CPPS makes clear that this multi-faceted condition affects patients’ quality of life on different levels. This highly variable syndrome does not imply a homogenous group of men with a common disease process. Stratifying classification systems to identify the individual clinical profile of every single patient are necessary. For adequate evaluation and treatment of patients with suspected CP/CPPS a systematic diagnostic assessment is of utmost importance.
3.2 Evaluation history
A detailed history is a mandatory first step in the evaluation of patients with suspected CP/CPPS. Several aspects of pain characteristics including location, radiation, quality of pain, severity, frequency, duration, triggers of pain and positive or negative modulators of pain need to be addressed. Given the multidimensional complexity of clinical manifestations, symptoms associated with CP/CPPS such as LUTS, sexual dysfunction and psychosocial factors should also be part of first assessment. A comprehensive history taking comprises the patient’s medication, surgical history, prior urinary tract infections, allergies and known comorbidities. It is important to stress, that these first information will guide the clinician towards adequate diagnostic steps to confirm or rule out the diagnosis of CP/CPPS. They will also considerably influence the individual treatment concept devised for the single patient.
3.3 Objective symptom appraisal
One milestone for the structured clinical evaluation of CP/CPPS patients was the introduction of a validated and standardized assessment tool and outcome measure. In 1999, the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) was developed and became thereafter the standard instrument for clinical practice and research [6], [8]. This self-administered questionnaire contains nine questions that are scored in three domains: pain, urinary symptoms and the impact on quality of life (total score 0–43). The main component is pain, which is captured in four items focusing on location, severity and frequency (score range 0–21). The urinary subscale consists of one irritative and one obstructive item (score range 0–10). The quality of life domain was captured in three items relating to the impact of symptoms on daily activities (score range 0–12). This psychometrically validated and discriminative tool demonstrated reliability, validity and adequate responsiveness to change [6], [30], [31]. Therefore, the NIH-CPSI became the generally accepted instrument in clinical trials providing appropriate primary endpoints [4], [5]. A six points decline in NIH-CPSI total score is considered a positive predictor of a meaningful treatment response [31]. In clinical practice it has proven invaluable for initial symptom detection and appraisal and most importantly, monitoring of treatment efficacy. Multiple validated translations of the NIH-CPSI are available [32], [33], [34], [35], [36], [37], [38], [39], [40]. Both NIH-CPSI total score and subscores for pain and quality of life were highly responsive if treatment was successful. Applying the NIH-CPSI as objective outcome measure in CP/CPPS patients revealed that pain intensity is a strong predictor of poor quality of life and it has the strongest impact on overall quality of life [8], [41].
Current guidelines recommend utilization of validated symptom-scoring instruments such as the NIH-CPSI for initial assessment of symptom severity and its impact on quality of life as well as for treatment monitoring (level of evidence 2b, grade of recommendation B) [42].
If medical history is suggestive of LUTS, the International Prostate Symptom Score (IPSS) [43] presents an appropriate validated outcome measure to be considered for basic evaluation and follow-up (level of evidence 2b, grade of recommendation B) [42]. Optional tools for evaluation and monitoring of sexual dysfunction, especially erectile dysfunction, include the five items version of the International Index of Erectile Function (IIEF-5) [44] or the Sexual Health Inventory for Men (SHIM) [45] (level of evidence 2b, grade of recommendation B) [42].
With its relevant impact on pain and patient adjustment in CP/CPPS, psychosocial symptoms (anxiety, depression or pain catastrophizing) should also be part of basic assessment (level of evidence 2b, grade of recommendation B) [42]. For psychosocial screening an optional array of validated questionnaires including the Public Health Questionnaire-2 (PHQ-2), Patient Health Questionnaire-9 (PHQ-9), the Generalised Anxiety Disorder-7 (GAD-7) or the Hospital Anxiety and Depression Scale (HADS) is available [46]. However in most clinical situations, focused questions in regard to rumination, magnification, helplessness, anxiety, stress social situation and coping suffice as a general clinic assessment.
3.4 Clinical phenotyping of CP/CPPS patients: UPOINTs
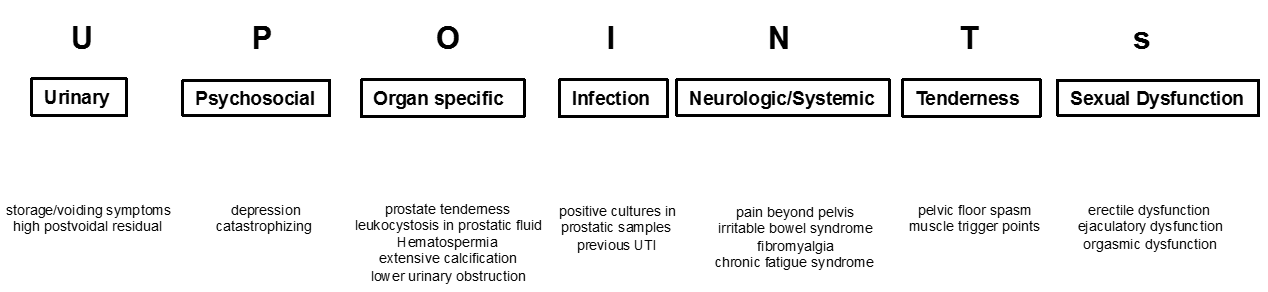
UPOINT represents a novel clinical phenotyping system for the management of CP/CPPS. This systematic stratification of clinical phenotypes reveals the individual clinical profile of every patient as a unique individual, provides guidance for further diagnostic steps and most importantly, suggests targets for a phenotypically directed multimodal treatment concept. The six UPOINT domains comprise U-rinary symptoms, P-sychological dysfunction, O-rgan-specific symptoms, I-nfection, N-eurologic/systemic conditions and T-enderness of muscles [12], [47], [48], [49]. UPOINT discriminates clinical phenotypes and the correlations between the number of positive domains and both symptom severity and symptom duration were confirmed [12]. Furthermore, in first clinical studies the number of positive UPOINT domains correlated with the total NIH-CPSI score, as well with its impact on quality of life [50], [51]. With sexual dysfunction afflicting 40% to 70% of men with CP/CPPS [14], [15], [16], [17], [18], a refined version of the UPOINT classification system including an additional domain for S-exual dysfunction was proposed. Clinical studies evaluating the correlation between positive UPOINTs domains and symptom severity revealed heterogeneous results [51], [52], [53], [54]. Most of the clinical studies reported improved consistency and validity of the modified UPOINTs version. However, all agree that sexual dysfunction domain should be addressed if present. Significant relief of symptoms and improved quality of life were achieved by a multi-modal treatment approach based on UPOINT classification. A prospective study enrolling a cohort of 100 CPPS patients with a median follow-up of 50 weeks investigated the clinical response to a phenotypically directed multi-modal treatment led by UPOINT [13]. The primary endpoint of a minimum six points reduction in total NIH-CPSI score was met in almost 84% of patients. All NIH-CPSI sub-domains including scores for pain, urinary symptoms and quality of life were significantly improved (each P˂0.0001). Two further independent clinical studies confirmed similar high responder rates when multi-modal therapy of CP/CPPS patients was guided by UPOINT [55], [56]. First clinical results are promising, but further randomized controlled trials are warranted for complete validation of the UPOINTs approach. Categorization according to UPOINTs is depicted in figure 1.
3.5 Physical examination
There are neither reliable physical findings nor specific diagnostic tests for CP/CPPS. The main objectives are to describe the current physical status suggestive of CP/CPPS and to exclude or identify differential diagnoses associated with pelvic pain. The clinical examination of abdomen, external genitalia, perineum and prostate is mandatory. On digital rectal examination, the prostate is evaluated for irregularities (asymmetry, induration, pain, tenderness), while examination of the rectum and pelvic floor muscles are helpful to determine myofascial trigger points and musculoskeletal dysfunction. The exact pathophysiological role of pelvic myofascial abnormalities still remains elusive in CP/CPPS, but its evaluation is considered helpful in treatment decisions [57], [58].
3.6 Laboratory tests
No specific laboratory test for the diagnosis of CP/CPPS is available. The 4-glass test as described by Meares and Stamey [59] provides information about the infectious origin of the condition, suggestive of chronic bacterial prostatitis (NIH category II), and confirms an inflammatory component, which allow for the classification of CP/CPPS into an inflammatory (NIH category IIIA) or noninflammatory type (NIH category IIIB). The clinical relevance of differentiating category IIIA from IIIB has not been definitively proven in clinical treatment trials. Evidence for chronic bacterial prostatitis in symptomatic patients necessitates adequate antimicrobial treatment, preferably with a fluorochinolone (level of evidence 2b, grade of recommendation B) [60]. Thus, microbiological localization cultures are the criterion standard to rule out chronic bacterial prostatitis and should be performed for evaluation (grade of recommendation A) (42). The detection of prostatic inflammation (e.g. quantitiy of leukocytes per high-power field in prostatic specimen) has no clear benefit for the management of CP/CPPS. While the complete 4-glass test requires the analysis of first voided urine, midstream urine, expressed prostatic secretion and post-prostate massage urine, a simpler 2-glass test is also possible as accurate screen for initial evaluation. Here, the investigation of pre- and post-prostate massage urine had strong concordance with the standard 4-glass test [61]. The clinical value of prostatic localization tests still remains controversial. Clinical studies failed to demonstrate a correlation between symptom severity and both leukocytes and bacterial counts in CP/CPPS patients [62]. Furthermore, one clinical study observed that positive localization cultures in prostatic specimen were similar between CP/CPPS patients and an asymptomatic age matched control group, while the control group presented also a high prevalence of leukocytes [63]. Randomized controlled trials evaluating the role of specific anti-inflammatory agents for the treatment of CP/CPPS type IIIA provided heterogeneous results [4], [5]. Of note, because of the additional expense in daily practice, the 4-glass test is only rarely used by urologists [64]. However, it is important to determine the culture status of the lower urinary tract before prescribing antibiotics.
Due to the low sensitivity and specificity compared to standard localization tests, culture and/or microscopic examination of semen are not recommended [60].
No reliable and validated biomarker is available for the management of CP/CPPS. Prostate-specific antigen (PSA) was analysed for its use as diagnostic parameter in CP/CPPS patients compared to case-controlled men. Due to insufficient sensitivity and specificity, PSA was proven to be an inappropriate biomarker [65]. The screening for accurate biomarkers in CP/CPPS is ongoing and first promising candidates warrant further studies to determine their value for diagnosis and treatment monitoring [66], [67], [68], [69], [70], [71], [72], [73], [74], [75].
3.7 Imaging techniques
No imaging procedure is recommended for basic evaluation [42], [46], [60]. Procedures should be selected for specific indications or to exclude differential diagnoses of abdominal or pelvic pain. Transrectal ultrasound may be considered in selected patients for investigation of suspected pathologies like intraprostatic abscess, calcification or dilatation of seminal vesicles. Cystoscopy or retrograde urethrography are optional diagnostic procedures to rule out bladder outlet obstruction. Urodynamic studies may be considered in selected patients with clinically significant LUTS. Computed tomography scans or magnetic resonance imaging of the abdominal or pelvic area may be considered in specific indications.
3.8 Follow-up
Management of CP/CPPS not only requires a structured basic assessment and a tailored multi-modal phenotypic directed therapy, but also regular follow-ups to re-evaluate efficacy and safety of the initiated treatment programme. Given the multi-dimensional complexity of symptoms and the known late clinical response to treatment modalities, a first evaluation appears reasonable after 6 weeks, unless there are no side effects or complications. For the management of this bothersome condition it is essential to explain to the patient the first results of diagnostic assessment, the reasons for the individualized therapy concept and most importantly, to inform the patient about a certain latency of treatment response.
A thorough re-evaluation is necessary to verify adherence to treatment and its therapeutic impact on the course of disease. Objective outcome measures as described above proved to be invaluable to monitor clinical response. According to clinical phenotypes and initial assessment, symptom-scoring questionnaires should be considered to determine the course of symptoms with time or treatment. In this regard, diagnostic tests used for first assessment should be repeated to monitor clinical response. For example, if patients reported LUTS before treatment, urodynamic studies are helpful to document improvement after therapy with α-blockers.
Based on regular follow-ups, the treatment concept can be monitored, modified, and optimized in a structured process. An accurate basic assessment and regular re-evaluations over time are considered essential aspects for successful management of CP/CPPS. It is important that both the urologist and the patient have realistic treatment goals, especially if cure is not possible.
4 Further research
The complex pathophysiology underlying CP/CPPS is still poorly understood. This has made the management of this condition very challenging for both physicians and patients. A plethora of clinical trials in the last decades with limited success mirrors our dilemma. Nevertheless, with the introduction of a generally accepted classification system and a validated outcome measure like the NIH-CPSI, research and clinical advances have progressed substantially in the last years. Future research objectives are to decipher the complex etiology of CP/CPPS. A reliable and accurate biomarker for diagnosis of CP/CPPS and evaluation of treatment response is not available. First candidates have been identified but their value for clinical use still needs to be determined [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]. The role of the lower urinary tract and prostate microbiome and its impact on symptom patterns over time is just emerging [76]. The understanding of central processes underlying CP/CPPS may contribute to the development of novel therapies.
Another focus is on the optimal management strategy. The systematic classification of clinical phenotypes according to UPOINTs and the resulting multi-modal treatment concept present another progress for the management of CP/CPPS in recent years. The phenotypic approach for management of this highly heterogeneous syndrome made clear that it is of utmost importance to fully outline the clinical picture of the patient with suspected CP/CPPS. Especially, the relevance of psychosocial risk factors started to receive attention, but with regard to basic assessment and therapy in the context of CP/CPPS the management of this particular aspect still needs to be addressed and clinically validated [77].
5 Conclusions
The management of CP/CPPS has always been a formidable task in clinical practice. It is a heterogeneous syndrome affecting significantly quality of life in many men. With recent scientific and clinical advances in the understanding of this debilitating condition, successful management seems possible now. No specific test for the definitive diagnosis of CP/CPPS is available. The main objective for evaluation is to exclude differential diagnoses associated with pelvic pain and to identify the individual clinical profile relevant for a tailored treatment concept. Therefore, systematic initial evaluation including a thorough patient history and comprehensive assessment of symptoms based on objective outcome measures and clinical phenotyping classification system is of utmost importance. Further diagnostic procedures are adapted to the patient. An effective multi-modal treatment concept relies on this accurate basic evaluation and regular follow-ups to monitor treatment response with the option for modification. A diagnostic algorithm for patients suggestive of CP/CPPS is shown in figure 2.
References
[1] Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999 Jul;282(3):236-7.[2] Bartoletti R, Cai T, Mondaini N, Dinelli N, Pinzi N, Pavone C, Gontero P, Gavazzi A, Giubilei G, Prezioso D, Mazzoli S, Boddi V, Naber KG; Italian Prostatitis Study Group. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: results of a multicenter case-control observational study. J Urol. 2007 Dec;178(6):2411-5; discussion 2415. DOI: 10.1016/j.juro.2007.08.046
[3] de la Rosette JJ, Hubregtse MR, Meuleman EJ, Stolk-Engelaar MV, Debruyne FM. Diagnosis and treatment of 409 patients with prostatitis syndromes. Urology. 1993 Apr;41(4):301-7. DOI: 10.1016/0090-4295(93)90584-W
[4] Cohen JM, Fagin AP, Hariton E, Niska JR, Pierce MW, Kuriyama A, Whelan JS, Jackson JL, Dimitrakoff JD. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review and meta-analysis. PLoS ONE. 2012;7(8):e41941. DOI: 10.1371/journal.pone.0041941
[5] Magistro G, Wagenlehner FM, Grabe M, Weidner W, Stief CG, Nickel JC. Contemporary Management of Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Eur Urol. 2016 Feb;69(2):286-97. DOI: 10.1016/j.eururo.2015.08.061
[6] Litwin MS, McNaughton-Collins M, Fowler FJ Jr, Nickel JC, Calhoun EA, Pontari MA, Alexander RB, Farrar JT, O'Leary MP. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999 Aug;162(2):369-75. DOI: 10.1016/S0022-5347(05)68562-X
[7] Zermann DH, Ishigooka M, Doggweiler R, Schmidt RA. Neurourological insights into the etiology of genitourinary pain in men. J Urol. 1999 Mar;161(3):903-8. DOI: 10.1016/S0022-5347(01)61802-0
[8] Wagenlehner FM, van Till JW, Magri V, Perletti G, Houbiers JG, Weidner W, Nickel JC. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur Urol. 2013 May;63(5):953-9. DOI: 10.1016/j.eururo.2012.10.042
[9] Shoskes DA, Landis JR, Wang Y, Nickel JC, Zeitlin SI, Nadler R; Chronic Prostatitis Collaborative Research Network Study Group. Impact of post-ejaculatory pain in men with category III chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2004 Aug;172(2):542-7. DOI: 10.1097/01.ju.0000132798.48067.23
[10] Propert KJ, McNaughton-Collins M, Leiby BE, O'Leary MP, Kusek JW, Litwin MS; Chronic Prostatitis Collaborative Research Network. A prospective study of symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome: the National Institutes of Health Chronic Prostatitis Cohort study. J Urol. 2006 Feb;175(2):619-23; discussion 623. DOI: 10.1016/S0022-5347(05)00233-8
[11] Samplaski MK, Li J, Shoskes DA. Clustering of UPOINT domains and subdomains in men with chronic prostatitis/chronic pelvic pain syndrome and contribution to symptom severity. J Urol. 2012 Nov;188(5):1788-93. DOI: 10.1016/j.juro.2012.07.036
[12] Shoskes DA, Nickel JC, Dolinga R, Prots D. Clinical phenotyping of patients with chronic prostatitis/chronic pelvic pain syndrome and correlation with symptom severity. Urology. 2009 Mar;73(3):538-42; discussion 542-3. DOI: 10.1016/j.urology.2008.09.074
[13] Shoskes DA, Nickel JC, Kattan MW. Phenotypically directed multimodal therapy for chronic prostatitis/chronic pelvic pain syndrome: a prospective study using UPOINT. Urology. 2010 Jun;75(6):1249-53. DOI: 10.1016/j.urology.2010.01.021
[14] Beutel ME, Weidner W, Brähler E. Der chronische Beckenschmerz und seine Komorbidität [Chronic pelvic pain and its comorbidity]. Urologe A. 2004 Mar;43(3):261-7. DOI: 10.1007/s00120-003-0521-2
[15] Lee SW, Liong ML, Yuen KH, Leong WS, Cheah PY, Khan NA, Krieger JN. Adverse impact of sexual dysfunction in chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008 Jan;71(1):79-84. DOI: 10.1016/j.urology.2007.08.043
[16] Magri V, Perletti G, Montanari E, Marras E, Chiaffarino F, Parazzini F. Chronic prostatitis and erectile dysfunction: results from a cross-sectional study. Arch Ital Urol Androl. 2008 Dec;80(4):172-5.
[17] Mehik A, Hellström P, Sarpola A, Lukkarinen O, Järvelin MR. Fears, sexual disturbances and personality features in men with prostatitis: a population-based cross-sectional study in Finland. BJU Int. 2001 Jul;88(1):35-8. DOI: 10.1046/j.1464-410x.2001.02259.x
[18] Trinchieri A, Magri V, Cariani L, Bonamore R, Restelli A, Garlaschi MC, Perletti G. Prevalence of sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome. Arch Ital Urol Androl. 2007 Jun;79(2):67-70.
[19] Liang CZ, Hao ZY, Li HJ, Wang ZP, Xing JP, Hu WL, Zhang TF, Ge WW, Zhang XS, Zhou J, Li Y, Zhou ZX, Tang ZG, Tai S. Prevalence of premature ejaculation and its correlation with chronic prostatitis in Chinese men. Urology. 2010 Oct;76(4):962-6. DOI: 10.1016/j.urology.2010.01.061
[20] Liang CZ, Zhang XJ, Hao ZY, Shi HQ, Wang KX. Prevalence of sexual dysfunction in Chinese men with chronic prostatitis. BJU Int. 2004 Mar;93(4):568-70. DOI: 10.1111/j.1464-410X.2003.04662.x
[21] Egan KJ, Krieger JN. Psychological problems in chronic prostatitis patients with pain. Clin J Pain. 1994 Sep;10(3):218-26. DOI: 10.1097/00002508-199409000-00008
[22] Ku JH, Kim SW, Paick JS. Quality of life and psychological factors in chronic prostatitis/chronic pelvic pain syndrome. Urology. 2005 Oct;66(4):693-701. DOI: 10.1016/j.urology.2005.04.050
[23] Nickel JC, Tripp DA, Chuai S, Litwin MS, McNaughton-Collins M, Landis JR, Alexander RB, Schaeffer AJ, O'Leary MP, Pontari MA, White P, Mullins C, Nyberg L, Kusek J; NIH-CPCRN Study Group. Psychosocial variables affect the quality of life of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2008 Jan;101(1):59-64. DOI: 10.1111/j.1464-410X.2007.07196.x
[24] Tripp DA, Nickel JC, Wang Y, Litwin MS, McNaughton-Collins M, Landis JR, Alexander RB, Schaeffer AJ, O'Leary MP, Pontari MA, Fowler JE Jr, Nyberg LM, Kusek JW; National Institutes of Health-Chronic Prostatitis Collaborative Research Network (NIH-CPCRN) Study Group. Catastrophizing and pain-contingent rest predict patient adjustment in men with chronic prostatitis/chronic pelvic pain syndrome. J Pain. 2006 Oct;7(10):697-708. DOI: 10.1016/j.jpain.2006.03.006
[25] Rodríguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009 Nov;182(5):2123-31. DOI: 10.1016/j.juro.2009.07.036
[26] Clemens JQ, Brown SO, Kozloff L, Calhoun EA. Predictors of symptom severity in patients with chronic prostatitis and interstitial cystitis. J Urol. 2006 Mar;175(3 Pt 1):963-6; discussion 967. DOI: 10.1016/S0022-5347(05)00351-4
[27] Dimitrakov J, Joffe HV, Soldin SJ, Bolus R, Buffington CA, Nickel JC. Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008 Feb;71(2):261-6. DOI: 10.1016/j.urology.2007.09.025
[28] Pontari MA. Chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis: are they related? Curr Urol Rep. 2006 Jul;7(4):329-34. DOI: 10.1007/s11934-996-0013-1
[29] Vicari E, La Vignera S, Arcoria D, Condorelli R, Vicari LO, Castiglione R, Mangiameli A, Calogero AE. High frequency of chronic bacterial and non-inflammatory prostatitis in infertile patients with prostatitis syndrome plus irritable bowel syndrome. PLoS ONE. 2011 Apr;6(4):e18647. DOI: 10.1371/journal.pone.0018647
[30] Litwin MS. A review of the development and validation of the National Institutes of Health Chronic Prostatitis Symptom Index. Urology. 2002 Dec;60(6 Suppl):14-8; discussion 18-9. DOI: 10.1016/S0090-4295(02)02296-3
[31] Propert KJ, Litwin MS, Wang Y, Alexander RB, Calhoun E, Nickel JC, O'Leary MP, Pontari M, McNaughton-Collins M; Chronic Prostatitis Collaborative Research Network (CPCRN). Responsiveness of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI). Qual Life Res. 2006 Mar;15(2):299-305. DOI: 10.1007/s11136-005-1317-1
[32] Cheah PY, Liong ML, Yuen KH, Lee S, Yang JR, Teh CL, Khor T, Yap HW, Krieger JN. Reliability and validity of the National Institutes of Health: Chronic Prostatitis Symptom Index in a Malaysian population. World J Urol. 2006 Feb;24(1):79-87. DOI: 10.1007/s00345-005-0037-z
[33] Collins MM, O'Leary MP, Calhoun EA, Pontari MA, Adler A, Eremenco S, Chang CH, Odom L, Litwin MS; Chronic Prostatitis Collaborative Research Network. The Spanish National Institutes of Health-Chronic Prostatitis Symptom Index: translation and linguistic validation. J Urol. 2001 Nov;166(5):1800-3. DOI: 10.1016/S0022-5347(05)65678-9
[34] El-Nashaar A, Fathy A, Zeedan A, Al-Ahwany A, Shamloul R. Validity and reliability of the arabic version of the National Institutes of Health Chronic Prostatitis Symptom Index. Urol Int. 2006;77(3):227-31. DOI: 10.1159/000094814
[35] Giubilei G, Mondaini N, Crisci A, Raugei A, Lombardi G, Travaglini F, Del Popolo G, Bartoletti R. The Italian version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur Urol. 2005 Jun;47(6):805-11. DOI: 10.1016/j.eururo.2004.12.025
[36] Karakiewicz PI, Perrotte P, Valiquette L, Benard F, McCormack M, Menard C, McNaughton Collins M, Nickel JC. French-Canadian linguistic validation of the NIH Chronic Prostatitis Symptom Index. Can J Urol. 2005 Oct;12(5):2816-23.
[37] Leskinen MJ, Mehik A, Sarpola A, Tammela TL, Järvelin MR. The Finnish version of The National Institutes Of Health Chronic Prostatitis Symptom Index correlates well with the visual pain scale: translation and results of a modified linguistic validation study. BJU Int. 2003 Aug;92(3):251-6.
[38] Monden K, Tsugawa M, Ninomiya Y, Ando E, Kumon H. [A Japanese version of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI, Okayama version) and the clinical evaluation of cernitin pollen extract for chronic non-bacterial prostatitis]. Nihon Hinyokika Gakkai Zasshi. 2002 May;93(4):539-47. DOI: 10.5980/jpnjurol1989.93.539
[39] Novotny C, Deves E, Novotny R, Rodrigues IK, Neves FS. Cultural adaptation of the National Institutes of Health--chronic prostatitis symptom index (NIH-CPSI)--to Brazilian spoken Portuguese: NIH-CPSI (Braz). Int Braz J Urol. 2013 Sep-Oct;39(5):683-91. DOI: 10.1590/S1677-5538.IBJU.2013.05.11
[40] Schneider H, Brähler E, Ludwig M, Hochreiter W, Collins MF, Eremenco S, Weidner W. Two-year experience with the german-translated version of the NIH-CPSI in patients with CP/CPPS. Urology. 2004 Jun;63(6):1027-30. DOI: 10.1016/j.urology.2004.02.002
[41] Tripp DA, Curtis Nickel J, Landis JR, Wang YL, Knauss JS; CPCRN Study Group. Predictors of quality of life and pain in chronic prostatitis/chronic pelvic pain syndrome: findings from the National Institutes of Health Chronic Prostatitis Cohort Study. BJU Int. 2004 Dec;94(9):1279-82. DOI: 10.1111/j.1464-410X.2004.05157.x
[42] Engeler D, Baranowski AP, Borovicka J, Dinis-Oliveira P, Elneil s, Hughes J, Messelink EJ, de C Williams AC. Guidelines on Chronic Pelvic Pain. European Association of Urology; 2016. Available from: http://uroweb.org/wp-content/uploads/EAU-Guidelines-Chronic-Pelvic-Pain-2016.pdf
[43] Badía X, García-Losa M, Dal-Ré R. Ten-language translation and harmonization of the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol. 1997;31(2):129-40.
[44] Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997 Jun;49(6):822-30.
[45] Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005 Jul-Aug;17(4):307-19. DOI: 10.1038/sj.ijir.3901327
[46] Rees J, Abrahams M, Doble A, Cooper A; Prostatitis Expert Reference Group (PERG). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int. 2015 Oct;116(4):509-25. DOI: 10.1111/bju.13101
[47] Nickel JC, Shoskes D. Phenotypic approach to the management of chronic prostatitis/chronic pelvic pain syndrome. Curr Urol Rep. 2009 Jul;10(4):307-12. DOI: 10.1007/s11934-009-0050-7
[48] Shoskes DA, Nickel JC. Classification and treatment of men with chronic prostatitis/chronic pelvic pain syndrome using the UPOINT system. World J Urol. 2013 Aug;31(4):755-60. DOI: 10.1007/s00345-013-1075-6
[49] Shoskes DA, Nickel JC, Rackley RR, Pontari MA. Clinical phenotyping in chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis: a management strategy for urologic chronic pelvic pain syndromes. Prostate Cancer Prostatic Dis. 2009;12(2):177-83. DOI: 10.1038/pcan.2008.42
[50] Hedelin HH. Evaluation of a modification of the UPOINT clinical phenotype system for the chronic pelvic pain syndrome. Scand J Urol Nephrol. 2009;43(5):373-6. DOI: 10.3109/00365590903164514
[51] Magri V, Wagenlehner F, Perletti G, Schneider S, Marras E, Naber KG, Weidner W. Use of the UPOINT chronic prostatitis/chronic pelvic pain syndrome classification in European patient cohorts: sexual function domain improves correlations. J Urol. 2010 Dec;184(6):2339-45. DOI: 10.1016/j.juro.2010.08.025
[52] Davis SN, Binik YM, Amsel R, Carrier S. Is a sexual dysfunction domain important for quality of life in men with urological chronic pelvic pain syndrome? Signs "UPOINT" to yes. J Urol. 2013 Jan;189(1):146-51. DOI: 10.1016/j.juro.2012.08.083
[53] Samplaski MK, Li J, Shoskes DA. Inclusion of erectile domain to UPOINT phenotype does not improve correlation with symptom severity in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2011 Sep;78(3):653-8. DOI: 10.1016/j.urology.2011.04.016
[54] Zhao Z, Zhang J, He J, Zeng G. Clinical utility of the UPOINT phenotype system in Chinese males with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a prospective study. PLoS ONE. 2013;8(1):e52044. DOI: 10.1371/journal.pone.0052044
[55] Guan X, Zhao C, Ou ZY, Wang L, Zeng F, Qi L, Tang ZY, Dun JG, Liu LF. Use of the UPOINT phenotype system in treating Chinese patients with chronic prostatitis/chronic pelvic pain syndrome: a prospective study. Asian J Androl. 2015 Jan-Feb;17(1):120-3. DOI: 10.4103/1008-682X.138189
[56] Magri V, Marras E, Restelli A, Wagenlehner FM, Perletti G. Multimodal therapy for category III chronic prostatitis/chronic pelvic pain syndrome in UPOINTS phenotyped patients. Exp Ther Med. 2015 Mar;9(3):658-666. DOI: 10.3892/etm.2014.2152
[57] Anderson RU, Wise D, Sawyer T, Chan C. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J Urol. 2005 Jul;174(1):155-60. DOI: 10.1097/01.ju.0000161609.31185.d5
[58] Fitzgerald MP, Anderson RU, Potts J, Payne CK, Peters KM, Clemens JQ, Kotarinos R, Fraser L, Cosby A, Fortman C, Neville C, Badillo S, Odabachian L, Sanfield A, O'Dougherty B, Halle-Podell R, Cen L, Chuai S, Landis JR, Mickelberg K, Barrell T, Kusek JW, Nyberg LM; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol. 2013 Jan;189(1 Suppl):S75-85. DOI: 10.1016/j.juro.2012.11.018
[59] Meares EM, Stamey TA. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968 Mar;5(5):492-518.
[60] Grabe M, Bartoletti R, Bjerklund Johansen TE, Cai T, Çek M, Köves B, Naber KG, Pickard RS, Tenke P, Wagenlehner F, Wullt B. Guidelines on Urological Infections. European Association of Urology; 2015. Available from: http://uroweborg/wp-content/uploads/19-Urological-infections_LR2pdf
[61] Nickel JC, Shoskes D, Wang Y, Alexander RB, Fowler JE Jr, Zeitlin S, O'Leary MP, Pontari MA, Schaeffer AJ, Landis JR, Nyberg L, Kusek JW, Propert KJ. How does the pre-massage and post-massage 2-glass test compare to the Meares-Stamey 4-glass test in men with chronic prostatitis/chronic pelvic pain syndrome? J Urol. 2006 Jul;176(1):119-24. DOI: 10.1016/S0022-5347(06)00498-8
[62] Schaeffer AJ, Knauss JS, Landis JR, Propert KJ, Alexander RB, Litwin MS, Nickel JC, O'Leary MP, Nadler RB, Pontari MA, Shoskes DA, Zeitlin SI, Fowler JE Jr, Mazurick CA, Kusek JW, Nyberg LM; Chronic Prostatitis Collaborative Research Network Study Group. Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort Study. J Urol. 2002 Sep;168(3):1048-53. DOI: 10.1016/S0022-5347(05)64572-7
[63] Nickel JC, Alexander RB, Schaeffer AJ, Landis JR, Knauss JS, Propert KJ; Chronic Prostatitis Collaborative Research Network Study Group. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003 Sep;170(3):818-22. DOI: 10.1097/01.ju.0000082252.49374.e9
[64] McNaughton Collins M, Fowler FJ Jr, Elliott DB, Albertsen PC, Barry MJ. Diagnosing and treating chronic prostatitis: do urologists use the four-glass test? Urology. 2000 Mar;55(3):403-7. DOI: 10.1016/S0090-4295(99)00536-1
[65] Nadler RB, Collins MM, Propert KJ, Mikolajczyk SD, Knauss JS, Landis JR, Fowler JE Jr, Schaeffer AJ, Alexander RB; Chronic Prostatitis Collaborative Research Network. Prostate-specific antigen test in diagnostic evaluation of chronic prostatitis/chronic pelvic pain syndrome. Urology. 2006 Feb;67(2):337-42. DOI: 10.1016/j.urology.2005.08.031
[66] Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998 Nov;52(5):744-9. DOI: 10.1016/S0090-4295(98)00390-2
[67] Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, Schaeffer AJ. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000 Dec 20;56(6):1025-9. DOI: 10.1016/S0090-4295(00)00844-X
[68] Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, Kreutzer DL. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002 Apr;59(4):603-8. DOI: 10.1016/S0090-4295(01)01597-7
[69] Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, Kreutzer DL. Interleukin-10 levels in seminal plasma: implications for chronic prostatitis-chronic pelvic pain syndrome. J Urol. 2002 Feb;167(2 Pt 1):753-6. DOI: 10.1016/S0022-5347(01)69139-0
[70] Watanabe T, Inoue M, Sasaki K, Araki M, Uehara S, Monden K, Saika T, Nasu Y, Kumon H, Chancellor MB. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011 Jul;108(2):248-51. DOI: 10.1111/j.1464-410X.2010.09716.x
[71] Shoskes DA, Wang H, Polackwich AS, Tucky B, Altemus J, Eng C. Analysis of Gut Microbiome Reveals Significant Differences between Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Controls. J Urol. 2016 Aug;196(2):435-41. DOI: 10.1016/j.juro.2016.02.2959
[72] Wei X, Zhang G, Yuan H, Ding X, Li S, Zhang X, Hou J. Detection and quantitation of soluble B7-H3 in expressed prostatic secretions: a novel marker in patients with chronic prostatitis. J Urol. 2011 Feb;185(2):532-7. DOI: 10.1016/j.juro.2010.09.104
[73] Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, Thumbikat P, Pope RM, Landis JR, Koch AE, Schaeffer AJ. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008 May;179(5):1857-61; discussion 1861-2. DOI: 10.1016/j.juro.2008.01.028
[74] Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007 Feb;51(2):524-33; discussion 533. DOI: 10.1016/j.eururo.2006.07.016
[75] Khadra A, Fletcher P, Luzzi G, Shattock R, Hay P. Interleukin-8 levels in seminal plasma in chronic prostatitis/chronic pelvic pain syndrome and nonspecific urethritis. BJU Int. 2006 May;97(5):1043-6. DOI: 10.1111/j.1464-410X.2006.06133.x
[76] Nickel JC, Stephens A, Landis JR, Chen J, Mullins C, van Bokhoven A, Lucia MS, Melton-Kreft R, Ehrlich GD; MAPP Research Network. Search for Microorganisms in Men with Urologic Chronic Pelvic Pain Syndrome: A Culture-Independent Analysis in the MAPP Research Network. J Urol. 2015 Jul;194(1):127-35. DOI: 10.1016/j.juro.2015.01.037
[77] Riegel B, Bruenahl CA, Ahyai S, Bingel U, Fisch M, Löwe B. Assessing psychological factors, social aspects and psychiatric co-morbidity associated with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) in men -- a systematic review. J Psychosom Res. 2014 Nov;77(5):333-50. DOI: 10.1016/j.jpsychores.2014.09.012




