Patient contributions to treatment decisions in BPS/IC
1 ICA-Deutschland e.V., Euskirchen, Deutschland
Abstract
BPS/IC patients are afflicted with a symptom complex consisting of increased micturition frequency, urinary urgency and bladder pain (frequency, urgency, pain). Because of the obscure aetiology it is important for patients to be involved in treatment decision concerning this condition. To achieve a satisfactory outcome out diagnosis and treatment, the ICA (Interstitial Cystitis Association) Germany e.V., www.ica-ev.de, has established an information platform for patients and physicians. This platform was designed to facilitate collaboration between clinicians and patients with the goal to improve therapeutic success. Patient self-management-strategies are highlighted with an emphasis on patient education, systematic documentation, nutritional adjustment, pain management and lifestyle changes including stress management.
Summary of recommendations
- Patient education should be key part of the treatment of BPS/IC. The best support a physician can offer is to act like a partner and make it understandable to the patient, that concerning this disease, there is no easy solution and no standard therapy plan that fits for every patient.
- A systematic documentation of symptoms and all circumstances which influence the state of health makes it easier to find possibilities to help. The patient should take his notes in corresponding diaries.
-
Considering and treating possible attendant symptoms in an early stage of BPS/IC can protect against the manifestation of companion diseases. Beside the basic therapy, there are many possibilities which might help to improve quality of life with BPS/IC. Therefore it is important for every patient to undergo trial and error method with recommended treatments and find out what works for him individually:
- Nutrition seems to affect the condition of nearly all patients. For this reason a controlled method for eliminating dietary sensitivities can be important.
- Sleep disorder prevention should be considered in any case. Those patients who are suffering from sleep disorder, often report an improvement by taking Vitamin B, Magnesium and Tryptophan.
- Bladder infection prevention is also very important, because additional bacterial infections and thus antibiotic therapies might cause further damage to the mucous membrane and intestinal flora. Anecdotal evidence shows that patients who tend to additional bacterial infections often benefit from D-mannose, birch leaves, goldenrod and bearberry.
- Receiving intestinal health should always be taken into account during the accompanying therapy especially when antibiotic treatments are unavoidable.
- Pain management strategies are indispensable for BPS/IC patients. Each patient should (in addition to a pharmacological therapy) learn to deal with his pain. Based on the experiences of patients, alternative treatments such as acupuncture, homeopathy, hypnosis and music therapy should be considered.
- Lifestyle change is important for all those suffering from a chronic disease and means that patients should learn to live according to their condition, to strive for their own quality of life. Many BPS/IC patients benefit from proven techniques for stress management like learning basic relaxation techniques, using meditation music and/or visualization, learning self-hypnosis and massage or doing suitable physical exercises such as yoga, low-impact aerobics, tai chi, pilates or walking. But also small adjustments, such as wearing warm and comfortable clothing, appropriate footwear or absorbing underwear can have a positive effect on the symptoms and well-being.
Introduction
Bladder pain syndrome/Interstitial cystitis (BPS/IC) is a disease characterized by a diverse and distressing symptom complex with unknown aetiology. It is important, therefore, to involve patients in decisions concerning diagnosis and treatment of this condition and thus achieve the best outcome of treatment [1]. To promote this approach in Germany, the ICA (Interstitial Cystitis Association) Germany e.V. has established a platform for patients and physicians that offer information in the form of booklets, brochures and flyers, along with telephone support and a web page. In the last 20 years, the ICA has circulated more than 510,000 information leaflets and brochures to patients and physicians. The telephone support line has been used by more than 75,000 patients in search of more detailed information on treatment of the disease. In addition, the ICA has held 226 patient meetings and over 200 continuing education events, as well as organizing eight international meetings for physicians. Opposed to a variety of other non-profit organizations, the ICA Germany e.V. is also a founding member of the Multinational Interstitial Cystitis Association (MICA). The goal of this diversified support service for patients and physicians is to enhance collaboration between the two groups and thus improve the provision of care and the success of treatment [2], [3]. With a goal to improving the situation, a unique healthcare study was designed to elicit more detailed information on the provision of care. The treatment course of patients with interstitial cystitis in Germany was recorded with the aid of a comprehensive questionnaire, which included questions on the disease, its treatment and impact on quality of life [4].
Even Paracelsus, the great Renaissance physician, purportedly told his patient, “You are the physician. We doctors are merely your assistants.” This suggests that to get the best outcomes of a therapy, patients should be involved in the care [5], [6]. In the case of interstitial cystitis this statement is quite appropriate. There is no treatment that benefits all patients. Therefore, multimodal therapy is required to achieve a satisfactory outcome [7], [8], [9]. In this context it is important to mention that comorbidities are common such as irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, allergies, autoimmune disorders, rheumatic diseases and migraine headaches, as well as mental health disorders such as depression (Figure 1). Based on these relations, it has been suggested that the disease may progress from an organ-specific phenotype (isolated bladder symptoms) to the regional (bladder symptoms plus irritable bowel syndrome,) and systemic phenotype (bladder symptoms plus irritable bowel syndrome plus fibromyalgia, chronic fatigue syndrome, migraines, etc.). This observation does not necessarily apply to all, but can be observed in a subset of patients [4], [10], [11], [12], [13], [14], [15]. Patient self-management can play a substantial role in the diagnosis. Patients should record their observations to keep track of important events [16].

(N=270; multiple responses possible [4]; illustration modified by ICA Germany)
Success of patient contributions to the treatment of interstitial cystitis
A collaborative relationship between the physician and patient, based on targeted guidance, can enhance the outcome of therapy. The physician likewise benefits from such a partnership, as both time and expenditure are reduced as a result of the patient accepting a fair share of the responsibility for the treatment programme. Numerous EPIC studies into lifestyle changes in patients have demonstrated that a modified lifestyle offers increased protection against a variety of diseases. To enhance the effect of treatment, a variety of aspects listed below has to be taken into account [17], [18].
Patient education
A physician has only a limited amount of time in which to care for a patient. It is therefore important to make the most out of the time available. The caregiver should consider providing the patient with a flyer that answers many of the patient's questions about interstitial cystitis, thereby saving valuable time. It is of fundamental importance that patients with interstitial cystitis take responsibility for improving their own health and quality of life. They need to accept that while their way of life needs to be modified, there are still many things that make life worthwhile.
Patients often have the impression that there is an easy solution for their problems, but anyone embarking on treatment with such expectations will be disappointed before treatment has even commenced. Therefore, physicians should help prevent such a situation by providing their patients with realistic goals while treating them as partners in the therapeutic process.
Educating the patient appropriately will help to avoid a range of psychosocial problems. Interstitial cystitis patients are often exposed to psychological stress such as sadness, a sense of guilt, and anxiety. These feelings can often exacerbate the symptoms. This, in turn, may affect issues such as travel, sleeping, family interactions, sexual function, and concentration (Figure 2). Consequently, the suicide rate amongst interstitial cystitis patients is higher than in the general population [4], [19].

(N=270; multiple responses possible [4]; illustration modified by ICA Germany)
Documentation
Systematic documentation of experiences related to the condition over a specified period, can be helpful in the creation of self-help options and self-help strategies. Micturition- and pain diaries are usually used as standard in the work-up of the diagnosis. In addition, all observations regarding food, physical activity, and other factors that affect the state of health should be documented so patients can find out what helps them personally and what they should avoid. As the process of searching for an appropriate therapy can demand a lot of patience and energy, interstitial cystitis patients should be prepared to continually accept and manage the changing circumstances of their illness.
Nutrition
Up to 90% of interstitial cystitis patients report that their condition is affected by what they eat. Their symptoms can deteriorate depending on their diet. Any aggravation of the symptoms varies from one individual to the other and may be influenced by comorbidities. The relationship between the ingestion of food and deterioration of symptoms appears to be part of the pathological mechanism. The mechanism is thought to be neural upregulation, epithelial dysfunction of the bladder and altered organ crosstalk. Survey results suggest that the symptoms are aggravated by the consumption of citrus fruits, tomatoes, artificial sweeteners, certain varieties of tea and coffee, carbonated or alcoholic drinks, and spicy foods. In contrast, calcium glycerolphosphate and sodium bicarbonate appear to allay the symptoms. For this reason, a controlled method for eliminating dietary sensitivities can be important when treating a patient [20], [21]. In the case of BPS/IC patients, special consideration should also be given to food allergies and intolerance, especially those involving atypical or delayed reactions which are difficult for the patient to recognize. Histamine intolerance plays a decisive role in the immune system. Histamine is a messenger molecule produced by the human body. All the mucous membranes are affected by histamine-related allergic reactions. Certain foods and medicinal products, including aspirin, diclofenac and amitriptyline, increase the release of histamine [22]. Type III allergies do not trigger any of the common immediate reactions, namely immune complex reactions mediated by IgM and IgG. In some individuals, the immune complexes bind to the epithelium, prompting immune complex degradation that result in complement activation in the small blood vessels. Symptoms occur 6–12 hours or longer after consumption and decrease the capacity for physical activity. This can be problematic because it is often difficult to relate the symptoms to the food that has triggered them. These reactions are often incorrectly interpreted by interstitial cystitis patients as deterioration in their symptoms or a side effect of interstitial cystitis therapy. Misinterpretations of this nature can significantly jeopardize the chances of therapeutic success. A modern test (KyberAllergoPlex 44) for reliably detecting type III allergies has now been available for some time. It can identify approx. 75% of the most significant food allergens involved in type III reactions [23], [24], [25].
Vitamin C
Vitamin C can promote the metabolism of histamine and enhance the stability of the connective tissue. It is recommended that interstitial cystitis patients take calcium ascorbate, which is non-acidic. Furthermore, vitamin C binds histamine and reduces its pro-inflammatory properties [26], [27].
Sleep disorder prevention
In treatment of BPS/IC doctors tend to focus on the main symptoms only. Consequently, other symptoms are often addressed too late with delayed treatment as consequence. Nearly all BPS/IC patients also suffer from urge symptoms and sleep disorders due to the pain. To avoid manifestations that might lead to psychological impairments and the need for drug therapy, it is important that the patients get recommendations for possibly helpful preventive measures at an early stage. It is important that the patients are aware that the options suggested may not help everyone. Each patient case is different and outcomes can vary. However, it is important for the patient to undergo trial and error method with recommended treatments and find what works for them individually.
Vitamin B
A deficiency of vitamin B6 appears to be associated with psychological distress and sleep disorders. This can be explained by the fact that pyridoxine, or vitamin B6, is a cofactor of 5-hydroxytryptophan decarboxylase, which is important to serotonin metabolism. It has been suggested that the activity of this vitamin is derived from its influence on the serotonin level in the brain, thereby regulating the circadian rhythm [28], [29]. Vitamin B12 shortens the sleep-wake cycle and positively influences sleep propensity. Above all, the bioactive form of vitamin B12, methylcobalamin, seems to have a positive impact on sleep disorders [30], [31], [32].
Magnesium
Magnesium is thought to promote cardiovascular health and reduce the risk of psychological issues such as despondency, restlessness, excitability, lack of concentration, noise sensitivity, rapid exhaustibility, sleep disorders, depression and amentia. Interstitial cystitis patients frequently report such psychological conditions. Therefore it is often recommended that interstitial cystitis patients take compounds of low acidity, such as magnesium aspartate or magnesium glycinate. Many patients report that taking magnesium before bedtime helps to improve sleep quality [33], [34], [35], [36], [37], [38], [39].
Tryptophan
Tryptophan is an essential aromatic amino acid which is not synthesised by the human body and needs to be ingested. Tryptophan is involved in serotonin and melatonin metabolism and is the precursor of both hormones and neurotransmitters. Serotonin and melatonin have an influence on the circadian rhythm, and are therefore thought to be important in the regulation of the sleep-wake cycle. Tryptophan deficiency can be caused by infections and autoimmune diseases, which are often observed as accompanying diseases in interstitial cystitis patients. For this reason, tryptophan substitution could also help to prevent psychological side effects of IC [4], [29], [40].
Bladder infection prevention
For patients suffering from BPS/IC, it is important to prevent bacterial infections and thus antibiotic therapies in order to avoid further changes of the mucous membrane and destruction of the intestinal flora.
The following substances are recommended for the treatment and prevention of urinary tract infections because of proven properties, long-term experience and/or extensive anecdotal evidence.
D-mannose
D-mannose is an aldohexose often found in polysaccharides, a component of plant cell walls. D-mannose is not metabolized to a significant degree and is mostly eliminated by the human body. D-mannose is bound to the pili of enterobacteria and so prevents adhesion of enterobacteria such as E. coli to the uroepithelium. D-mannose is reported to be an effective compound for preventing bacterial urinary tract infections with minimal side effects [41].
Birch leaves
(Betula pendula)
Birch leaves contain triterpenes, flavonoids, saponins and vitamin C, which exert an anti-inflammatory and aquaretic effect. The diuretic effect is probably caused by the flavonoids and supported by vitamin C. The leaves of the silver birch tree are used in the form of tea or oral medicine which is indicated for the prevention of urinary tract infections [42], [43], [44], [45].
Goldenrod
(Solidago virgaurea; Solidago canadensis; Solidago gigantea)
In one case study, a combination of goldenrod with alternative treatments such as magnesium produced a decrease in recurrent urinary tract symptoms. The pharmaceutically active ingredient can be found in the overground shoots of the plant, where saponins and flavonoids presumably exert anti-inflammatory and aquaretic activity. Goldenrod also appears to have bacteriostatic and diuretic effects. It is used as an oral medication and can help prevent or treat lower urinary tract disorders [46], [47].
Bearberry
In combination with dandelion extract, bearberry can be used as a prophylactic treatment for recurrent lower urinary tract infections. Phenol glycosides in bearberry have an antiseptic effect on the organs of the urinary tract, and also exert a bacteriostatic effect. Bearberry is administered orally. Only the leaves are pharmacologically active and should prove beneficial in fighting common bacteria found in urinary tract infections. It is also presumed that the efficacy of the compound is greater in alkaline urine. Therefore, foods that increase the acidity of the urine should be avoided during treatment. Given its toxic effect on the liver, bearberry should not be used prophylactically [48], [49], [50], [51], [52].
Intestinal health
The intestinal flora is important to the immune system, and therefore also plays a part in the development of bladder infections. Antibiotic treatments can cause severe damage to the intestinal flora. Unfortunately, interstitial cystitis patients are frequently treated with antibiotics, despite the absence of documented infections. Treatment with antibiotics can have unwanted effects on the microbiome. The detrimental effect depends on the type of antibiotic, dosing and length of treatment. This is why a targeted rehabilitation of the intestinal flora can be particularly important for interstitial cystitis patients. IC patients should therefore be informed about the causal relationship and possibilities which might help to keep their intestinal flora healthy – e.g. by intake of probiotics [53], [54], [59].
Pain management
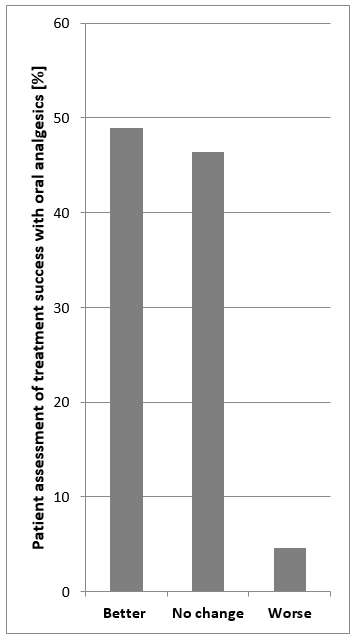
Healthcare providers recommend a variety of pain medications in BPS/IC. Assessments of the therapeutic success of oral analgesics made by patients reveal that almost 50% report an improvement in pain, whilst 46% report no change and 4% of patients report a worsening of their pain (Figure 3). This suggests that self-management of pain also contributes significantly to the successful treatment of symptoms. Aside from pharmacological therapy, patients should also seek options that will enable them to best deal with their pain. Pain management should be based on the experience of the patient. In addition, alternative treatments such as acupuncture, homeopathy and hypnosis might be considered as other means of reducing pain. Music therapy is another way to alleviate pain. The right music can soothe the soul, overwriting an individual’s “pain memory” with music to create positive impressions and experiences [4], [8], [9], [55].
Lifestyle change
Patients need to accept interstitial cystitis as an illness and change their lifestyle accordingly, discovering for themselves what they can and cannot do. With the doctor’s support and their own input, any chosen treatment should offer an improvement in quality of life.
Forty-two percent of interstitial cystitis patients recognize that stress plays a part in the flare-up of their condition. Having to cope with interstitial cystitis in itself can be a source of stress. Therefore, patients should make appropriate stress management a key part of their lives. Stress levels can be reduced by learning basic relaxation techniques, using meditation music and/or visualization, learning self-hypnosis and massage. Complaints often have a direct impact on the stress limit. Therefore alternative therapy methods can also be helpful: Biofeedback training and physical therapies, such as massage, can help to relieve cramps in the pelvic floor muscles and improve symptoms of pain and urgency. A bladder training program, requiring patients to carefully document their progress, is a successful way for interstitial cystitis patients to manage their stress.
Physical exercise is another important measure for reducing stress levels in interstitial cystitis patients. Many forms of exercise can improve general well-being and specific interstitial cystitis symptoms. Sports such as yoga, low-impact aerobics, tai chi, pilates and walking are particularly suitable for interstitial cystitis patients [8], [9], [16], [56].
Surprisingly, the characteristics of the patient’s clothing can have a significant impact on symptoms. Comfortable clothing makes frequent visits to the toilet significantly easier. Tight pants/undergarments may have profound effects on bladder pain as well as vulvar pain in those who suffer from vulvodynia.
In case of urge incontinence, absorbent underwear can help to relax and contribute to a normal daily routine. Warm clothing can prevent symptoms from being triggered by the cold. Appropriate foot wear is important, moreover, in the prevention of additional pelvic floor tension. High heels can trigger foot reflexology pressure points, which could exacerbate symptoms [16], [57], [58].
Conclusion
The first phase of the process can entail providing patients with informational materials that help them to learn about their condition and the available treatment options independently prior to their next appointment. It could also be beneficial to give them tasks to take home. This will encourage patients to develop a sense of responsibility and the feeling that they are being taken seriously. At the same time, they will also feel obligated to complete their “homework”. This is the first step toward patient cooperation and will save physicians a lot of valuable time. Training is important, in that it encourages patients to assume responsibility for their situation right from the start. Urging patients to record accurate, detailed information, and showing appreciation for their input, is essential. This will also save a lot of valuable time, provide them with motivation and a sense of self-reliance, and pave the way for a satisfactory therapeutic outcome. It is also important to encourage and motivate interstitial cystitis patients to pay attention to their diet. Information about the ways in which they can manage their pain is paramount. Regular, appropriate exercise can provide patients with physical and mental strength, and improve the success of treatment over the long term [19] Patients who are adherent and compliant will be easier to work with, saving valuable treatment time. There are many factors that influence the treatment of interstitial cystitis and can improve a patient’s individual situation. Nevertheless, further medical research is needed before those affected can be offered a treatment for the causes of the condition. Greater awareness and improvement in the quality of care are no doubt essential for everyone involved.
References
[1] Wein AJ, Hanno PM, Gillenwater JY. Interstitial Cystitis: An Introduction to the Problem. In: Hanno PM, Staskin DR, Krane RJ, Wein AJ, editors. Interstitial Cystitis. London: Springer; 1990. pp 3-15. DOI: 10.1007/978-1-4471-3293-6_1[2] ICA Deutschland eV. Available from: http://www.ica-ev.de/
[3] Multinational Interstitial Cystitis Association. Available from: www.mica-online.org
[4] Jocham D, Froehlich G, Sandig F, Ziegler A. Die Versorgungssituation von Patienten mit interstitieller Zystitis in Deutschland : Ergebnisse einer Umfrage unter 270 Betroffenen [The care situation of patients with interstitial cystitis in Germany: results of a survey of 270 patients]. Urologe A. 2013 May;52(5):691-702. DOI: 10.1007/s00120-013-3130-8
[5] Cousins N. Anatomy of an Illness as Perceived by the Patient: Reflections on Healing and Regeneration. New York: WW Norton & Company; 1979.
[6] Stillman JM. Theophrastus Bombastus Von Hohenheim Called Paracelsus: His Personality and Influence as Physician, Chemist and Reformer. Chicago, London: Open court publishing Company; 1920.
[7] Kahn B, Lombardi T. Interstitial cystitis: Simplified diagnosis and treatment. 2016. Available from: http://contemporaryobgyn.modernmedicine.com/contemporary-obgyn/news/interstitial-cystitis-simplified-diagnosis-and-treatment
[8] Atchley MD, Shah NM, Whitmore KE. Complementary and alternative medical therapies for interstitial cystitis: an update from the United States. Transl Androl Urol. 2015 Dec;4(6):662-7. DOI: 10.3978/j.issn.2223-4683.2015.08.08
[9] Pang R, Ali A. The Chinese approach to complementary and alternative medicine treatment for interstitial cystitis/bladder pain syndrome. Transl Androl Urol. 2015 Dec;4(6):653-61. DOI: 10.3978/j.issn.2223-4683.2015.08.10
[10] Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997 May;49(5A Suppl):52-7.
[11] Clemens JQ, Meenan RT, O'Keeffe Rosetti MC, Kimes TA, Calhoun EA. Case-control study of medical comorbidities in women with interstitial cystitis. J Urol. 2008 Jun;179(6):2222-5. DOI: 10.1016/j.juro.2008.01.172
[12] Warren JW, Howard FM, Cross RK, Good JL, Weissman MM, Wesselmann U, Langenberg P, Greenberg P, Clauw DJ. Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology. 2009 Jan;73(1):52-7. DOI: 10.1016/j.urology.2008.06.031
[13] Rodríguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009 Nov;182(5):2123-31. DOI: 10.1016/j.juro.2009.07.036
[14] Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010 Oct;184(4):1358-63. DOI: 10.1016/j.juro.2010.06.005
[15] Clemens JQ, Elliott MN, Suttorp M, Berry SH. Temporal ordering of interstitial cystitis/bladder pain syndrome and non-bladder conditions. Urology. 2012 Dec;80(6):1227-31. DOI: 10.1016/j.urology.2012.06.059
[16] Vahlensieck W. Interstitielle Cystitis und Diät. In: ICA-Deutschland eV, editor. Interstitielle Cystitis the state of the Art. Köln: Biermann Verlag; 2002.
[17] Lotz W. Selbsthilferatgeber für IC-Patientinnen und IC Patienten. 2015. Available from: https://www.ica-ev.de/downloads/Selbsthilferatgeber_IC_Patienten.pdf
[18] Wördehoff A. Die Möglichkeiten der alternativen IC-Therapie. In: ICA Deutschland e.V., editors. Interstitielle Cystitis. The State of the Art.Köln: Biermann; 2002. p. 85-88.
[19] Kanter G, Volpe KA, Dunivan GC, Cichowski SB, Jeppson PC, Rogers RG, Komesu YM. Important role of physicians in addressing psychological aspects of interstitial cystitis/bladder pain syndrome (IC/BPS): a qualitative analysis. Int Urogynecol J. 2017 Feb;28(2):249-256. DOI: 10.1007/s00192-016-3109-2
[20] Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome (IC/BPS) and comorbid conditions. BJU Int. 2012 Jun;109(11):1584-91. DOI: 10.1111/j.1464-410X.2011.10860.x
[21] Vahlensieck W. Die stationäre urologische Rehabilitation bei der interstitiellen Cystitis. In: ICA-Deutschland eV, editor. Interstitielle Cystitis the state of the Art. Köln: Biermann Verlag; 2002.
[22] Heßdörfer E. Neue Therapieoptionen bei interstitieller Zystitis Histamin setzt Blase zu. Uro-News. 2015; 19(12):40-1. DOI: 10.1007/s00092-015-0883-y
[23] Vervloet D, Durham S. Adverse reactions to drugs. BMJ. 1998 May 16;316(7143):1511-4. DOI: 10.1136/bmj.316.7143.1511
[24] Schnyder B, Pichler WJ. Mechanisms of drug-induced allergy. Mayo Clin Proc. 2009 Mar;84(3):268-72. DOI: 10.1016/S0025-6196(11)61145-2
[25] Brandtzaeg PE. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann N Y Acad Sci. 2002 May;964:13-45. DOI: 10.1111/j.1749-6632.2002.tb04131.x
[26] Schweizerische Interessensgemeinschaft Histamin-Intoleranz. Medikamente. Available from: http://www.histaminintoleranz.ch/de/therapie_medikamente.html
[27] Pfeiffer CC. Nährstoff-Therapie bei psychischen Störungen. Heidelberg: Karl F. Haug Verlag; 1986.
[28] Baldewicz T, Goodkin K, Feaster DJ, Blaney NT, Kumar M, Kumar A, Shor-Posner G, Baum M. Plasma pyridoxine deficiency is related to increased psychological distress in recently bereaved homosexual men. Psychosom Med. 1998 May-Jun;60(3):297-308. DOI: 10.1097/00006842-199805000-00016
[29] Halberg F, Stephens AN. Susceptibility to ouabain and physiologic circadian periodicity. Proc Minn Acad Sci. 1959;27:139–143.
[30] Okawa M, Mishima K, Nanami T, Shimizu T, Iijima S, Hishikawa Y, Takahashi K. Vitamin B12 treatment for sleep-wake rhythm disorders. Sleep. 1990 Feb;13(1):15-23. DOI: 10.1093/sleep/13.1.15
[31] Ohta T, Ando K, Iwata T, Ozaki N, Kayukawa Y, Terashima M, Okada T, Kasahara Y. Treatment of persistent sleep-wake schedule disorders in adolescents with methylcobalamin (vitamin B12). Sleep. 1991 Oct;14(5):414-8.
[32] Lichstein KL, Payne KL, Soeffing JP, Heith Durrence H, Taylor DJ, Riedel BW, Bush AJ. Vitamins and sleep: an exploratory study. Sleep Med. 2007 Dec;9(1):27-32. DOI: 10.1016/j.sleep.2006.12.009
[33] Matsuzaki H, Katsumata S, Kajita Y, Miwa M. Magnesium deficiency regulates vitamin D metabolizing enzymes and type II sodium-phosphate cotransporter mRNA expression in rats. Magnes Res. 2013 Apr-Jun;26(2):83-6. DOI: 10.1684/mrh.2013.0341
[34] Barragán-Rodríguez L, Rodríguez-Morán M, Guerrero-Romero F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res. 2008 Dec;21(4):218-23.
[35] Classen HG. Systemic stress, magnesium status and cardiovascular damage. Magnesium. 1986;5(3-4):105-10.
[36] Fehlinger R. Magnesium und tetanisches Syndrom. Magnesium Bull. 1980;2:40-7.
[37] Held K, Antonijevic IA, Künzel H, Uhr M, Wetter TC, Golly IC, Steiger A, Murck H. Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry. 2002 Jul;35(4):135-43. DOI: 10.1055/s-2002-33195
[38] Murck H. Ketamine, magnesium and major depression--from pharmacology to pathophysiology and back. J Psychiatr Res. 2013 Jul;47(7):955-65. DOI: 10.1016/j.jpsychires.2013.02.015
[39] Schmidt J. Magnesiumorotat. Dtsch Apotheker Z. 1998;138:66-70.
[40] Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun. 2002 Oct;16(5):590-5. DOI: 10.1016/S0889-1591(02)00006-5
[41] Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014 Feb;32(1):79-84. DOI: 10.1007/s00345-013-1091-6
[42] Hoffmann D. Medical Herbalism: The Science and Practice of Herbal Medicine. Rochester: Healing Arts Press; 2010. p. 534.
[43] Künkele U, Lohmeyer TR. Heilpflanzen und Kräuter. Köln: Parragon; 2007.
[44] Thurzova L. Lexikon der Heilpflanzen. Köln: Lingen Verlag; 1986.
[45] Mayer J, Uehleke B, Saum K. Handbuch der Klosterheilkunde. München: Zabert Sandmann; 2009.
[46] Mansour A, Hariri E, Shelh S, Irani R, Mroueh M. Efficient and cost-effective alternative treatment for recurrent urinary tract infections and interstitial cystitis in women: a two-case report. Case Rep Med. 2014;2014:698758. DOI: 10.1155/2014/698758
[47] Aschkenazi SO, Sand PK. Alternative Therapies for Urinary Urgency Incontinence: Acupuncture and Herbology. In: Continence: Current concepts and treatment strategies. London: Springer; 2009. pp. 203-215.
[48] Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Current therapeutic research. 1993;53(4):441-443. DOI: 10.1016/S0011-393X(05)80204-8
[49] Frohne D. Untersuchungen zur Frage der harndesinfizierenden Wirkungen von Bärentrubenblatt-Extrakten [The urinary disinfectant effect of extract from leaves uva ursi]. Planta Med. 1970 Jan;18(1):1-25. DOI: 10.1055/s-0028-1099743
[50] Kedzia B, Wrociński T, Mrugasiewicz K, Gorecki P, Grzewińska H. Przeciwbakteryjne działanie moczu zawierajacego produkty metabolizmu arbutyn [Antibacterial action of urine containing products of arbutin metabolism]. Med Dosw Mikrobiol. 1975;27(3):305-14.
[51] Tyler VE. Herbs of Choice. Philadelphia: Haworth Pr Inc; 1994.
[52] Leung AY. Encyclopedia of Common Natural Ingredients used in Food, Drugs, and Cosmetics. 2nd ed. New York: Wiley; 1996.
[53] Rafii F, Sutherland JB, Cerniglia CE. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag. 2008 Dec;4(6):1343-58. DOI: 10.2147/TCRM.S4328
[54] Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, de Vos WM. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016 Jan 26;7:10410. DOI: 10.1038/ncomms10410
[55] Bernatzky G. Arten der Schmerzbehandlung: Musiktherapie. Schmerznetz Österreich. Österreichische Schmerzgesellschaft. 2012.
[56] Wördehoff A. Die Möglichkeit der alternativen IC-Therapie. In: ICA-Deutschland eV, editor. Interstitielle Cystitis the state of the Art. Köln: Biermann Verlag; 2002. pp. 85-88.
[57] Whitmore KE. Complementary and alternative therapies as treatment approaches for interstitial cystitis. Rev Urol. 2002;4 Suppl 1:S28-35.
[58] Interstitial Cystitis Association. Available from: https://www.ichelp.org/
[59] Hiergeist A, Gessner A. Clinical implications of the microbiome in urinary tract diseases. Curr Opin Urol. 2017 Mar;27(2):93-98. DOI: 10.1097/MOU.0000000000000367




