Observational study over 8-year period evaluating microbiological characteristics and risk factor for isolation of multidrug-resistant organisms (MDRO) in patients with healthcare-associated infections (HAIs) hospitalized in a urology ward
Javier Gil-Moradillo 1
Alejandro González-Díaz 1
Pablo Abad-López 1
Rocu00edo Santos-Pérez de la Blanca 1
Mario Hernández-Arroyo 1
Helena Peña-Vallejo 1
Julio Téigell-Tobar 1
Cristina Calzas-Montalvo 1
Prado Caro-González 1
Natalia Miranda-Utrera 1
Ángel Tejido-Sánchez 1
1 Department of Urology, Health Research Institute i+12, Hospital Universitario 12 de Octubre, Madrid, Madrid, Spain
Abstract
Objective: To analyze, in a urology ward, the prevalence and characteristics of healthcare-associated infections (HAIs) due to multidrug-resistant organisms (MDRO).
Methods: We carried out an observational study from 2012 to 2019, evaluating MDRO among patients with HAIs, who were hospitalized in the urology ward. MDRO include Pseudomonas spp., resistant to at least three antibiotic groups, extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae or those resistant to carbapenems, and Enterococcus spp. resistant to vancomycin.
Results: Among patients with HAIs, MDRO were isolated in 100 out of 438 (22.8%) positive cultures. Univariate and multivariate analyses reported that prior urinary tract infection (UTI) [OR 2.45; 95% CI 1.14–5.36; p=0.021] and immunosuppression [OR 2.13; 95% CI 1.11–4.10; p=0.023] were risk factors for MDRO. A high prevalence of MRDO was found in patients with a catheter in the upper urinary tract; 27.6% for double-J stent, 29.6% in those with a nephrostomy tube, and 50% in those with a percutaneous internal/external nephroureteral (PCNU) stent. MDRO were isolated in 28.4% of cultures with Enterobacteriaceae (23.8% and 44.7% in those with E. coli and Klebsiella spp.); 7% of Enterobacteriaceae showed resistance to carbapenems (1.3% and 10% for E. coli and Klebsiella spp., respectively). Three out of 80 Enterococcus spp. were vancomycin-resistant. The rate of Pseudomonas aeruginosa resistant to at least three antibiotic groups was 36.3%.
Conclusions: The isolation of MDRO, in up to 25% of positive cultures in a urology ward, constitutes a challenge for the selection of antibiotics. MDRO are more common in immunosuppressed patients, those with previous UTIs, and those with a catheter in the upper urinary tract.
1 Introduction
The prevalence of multidrug-resistant organisms (MDRO) has been increasing worldwide in recent years [1], [2]. Isolation from MDRO is of utmost importance in patients with healthcare-associated infections (HAIs) [3], [4], [5]. It is a public health warning that, far from being controlled, this seems to be one of the main challenges for medicine in the 21st century. It is a problem that concerns all physicians and healthcare workers who manage patients with infections and use antibiotics. It is also necessary to bear in mind that when MDRO is isolated, there is limited availability of antibiotics to be prescribed [1], [6]. Thus, it seems of increasing importance for the detection of risk factors for HAIs and the isolation of MDRO to develop specific protocol and stewardship programs.
The prevalence of MDRO has been analyzed in some epidemiological studies [7]. However, data on the prevalence of MDRO in patients admitted to a urology service are limited. The main evaluation of MDRO in urology has been carried out by the GPIU study [4], [5], [8]. Urological patients have specific characteristics and specific risk factors, such as urinary catheters and surgery during hospitalization. Therefore, an adequate knowledge of risk factors, microbiological and resistance patterns for isolation of MDRO may allow select appropriate empiric antibiotic therapy and optimize outcomes [9]. Our purpose was to analyze the prevalence and characteristics of HAIs due to MDRO in patients admitted to a urology ward.
2 Methods
We have carried out an observational study from January 2012 to December 2019, evaluating the prevalence and risk factors for isolation of MDRO in patients with HAIs admitted in a urology ward. The study collected information of all patients admitted in the urology ward from scheduled admission or the emergency department. Pediatric patients and those who underwent kidney transplantation are not included, since they are admitted to the pediatric urology and nephrology departments, respectively.
We collected demographic data of all the patients admitted to our department, such as age, gender, comorbidities such as arterial hypertension, diabetes mellitus, cardiovascular disease, liver disease, immunosuppression, and health condition using the American Society of Anesthesiologists’ (ASA) score. Regarding urological risk factors, we collected urinary lithiasis, previous urinary tract infection (UTI), a urinary catheter (urethral or in the upper urinary tract, during hospitalization or placed prior to admission). The prevalence of infections was assessed and defined clinically regardless of the results of the cultures. Healthcare-associated infections (HAIs) are defined according to the criteria of the Centers for Disease Control and Prevention as infection diagnosed 48 hours after admission or those with any of the following criteria: received parenteral treatment, specialized wound care, hemodialysis, or intravenous chemotherapy; those admitted to an acute hospitalization ward for at least two days in the last 90 days, or institutionalized in a residence or long-stay center.
Multidrug resistance was defined according to the ECDC and CDC definitions of multidrug resistance (MDRO), extensive drug resistance (XDR) and pan-drug resistance (PDR). MDRO was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories, XDR was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e. bacterial isolates remain susceptible to only one or two categories) and PDR was defined as non-susceptibility to all agents in all antimicrobial categories [10]. We use the definition recommended by the ECDC and CDC which includes extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae or those resistant to carbapenems, Enterococcus spp. resistant to vancomycin, and Methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa resistant to more than three classes of antimicrobial agents. Pathogen identification, antimicrobial susceptibility testing and screening for ESBL and carbapenemase-producing bacteria were performed according to the cut-off point of resistance set by EUCAST (https://www.eucast.org). In brief, specimens were collected and processed following conventional microbiological procedures for correct management of clinical samples. Blood agar and MacConkey agar media were used. Identification and susceptibility tests were determined using the MicroScan® panel. The initial screen test to indicate ESBL production was done using cefotaxime and clavulanic acid. The confirmatory test was carried out by using the double disk synergy method with the determination of the MIC testing ceftriaxone or ceftazidime and ceftriaxone/ceftazidime in combination with clavulanic acid. E-test (Epsilon test) was also performed in those cases in which results were not conclusive. Carbapenemase-producing bacteria was considered to be any enterobacteria in which the values of the minimum inhibitory concentrations of at least one carbapenem (imipenem, meropenem, doripenem, or ertapenem) were equal to or higher than the cut-off point of resistance. The type of carbapenemase-producing bacteria was also reported.
The type of HAIs was reported including healthcare-associated urinary tract infections (HAUTIs) or other types of HAIs such as surgical site infection, intraabdominal abscess, and vascular catheter associated infections. We also recorded if MDRO were isolated in blood, urine cultures, or samples from surgical site or abscess. The analysis included microbiological characteristics and risk factors for isolation of MDR microorganisms in urine or blood cultures. As the study was carried out in a period of 8 years, we also review the evolution over time.
Data were presented mainly as frequencies expressed as a percentage in categorical variables; for the quantitative variable, data were reported as mean and standard deviation. Risk factors for isolation of MDRO in patients with HAIs were assessed by univariate analysis using Chi-square tests or Fisher’s test for categorical variables and Student’s t-test for continuous variables. A multivariate analysis was then performed, including those variables with a p<0.1 in the univariate analysis, to find independent risk factors for the development of infection due to MDRO. Data are presented as frequencies, as well as odds ratio (OR) with 95% confidence interval (95% CI). The p<0.05 are considered statistically significant. The data has been collected and analyzed with the Statistical Package for Social Sciences version 23.0 (SPSS Inc., Chicago, IL., USA).
3 Results
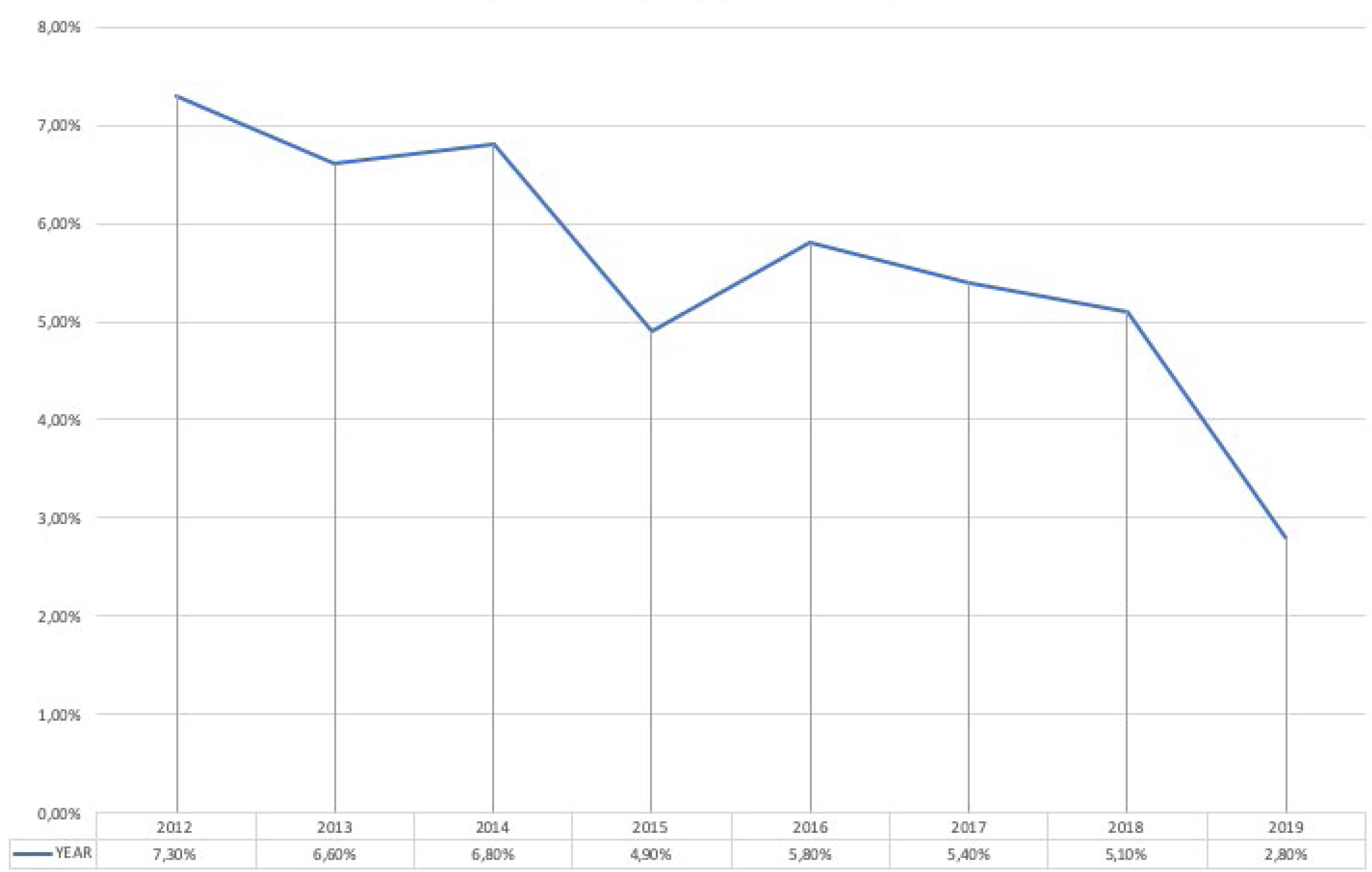
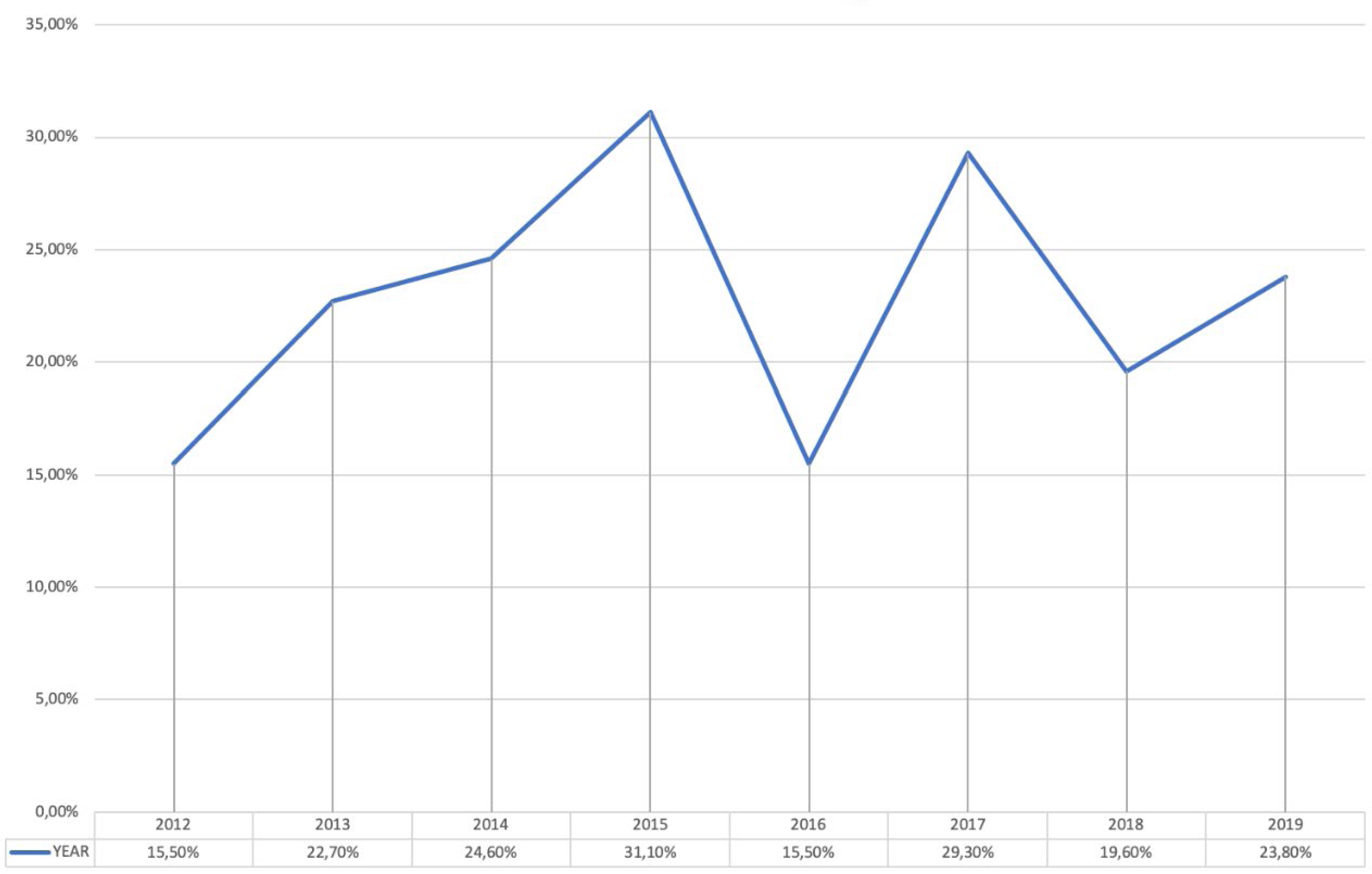
The results indicated that 778 (5.5%) out of 14,224 patients admitted to the urology ward between 2012 and 2019 reported HAIs, with a prevalence that decreases from 7.3% in 2012 to 2.8% in 2019. The evolution of the prevalence of HAIs is shown in Figure 1. No microorganisms were isolated in 28.4% of patients. One microorganism was isolated in 42.9%. Several microorganisms were isolated in 19.7%. No culture was available or the result showed contamination in 9%. In patients with HAIs with positive cultures, the prevalence of MDRO microorganisms was 22.8%, with figures ranging from 15.5% in 2012 and 2016 to 31.1% in 2015. The evolution of the prevalence of MDRO is shown in Figure 2. HAUTIs were the most common types of HAIs (71.5%), followed by surgical site infections and intraabdominal abscesses, 17.5% and 11%, respectively. In patients with isolation of MDRO, the types of infections were HAUTIs in 81.7%, surgical site infections in 12.5%, and intraabdominal abscess in 5.8%. MDRO were mainly isolated from urine cultures (59%), followed by blood cultures in 22.3%, and samples from surgical site or intraabdominal abscess in 18.7%.
In patients with HAIs and a urinary catheter in the upper urinary, the prevalence of isolation of MDRO was 27.6% for those with a double-J stent, 29.6% for those with a nephrostomy tube, and up to 50% in those with a percutaneous internal/external nephroureteral (PCNU) stent. The univariate analysis reported a higher prevalence of MDRO isolation in patients with arterial hypertension [OR 1.73; 95% CI 1.08–2.74; p=0.020], previous UTIs [OR 2.50; 95% CI 1.19–5.28; p=0.013] and immunosuppression [OR 2.25; 95% CI 1.19–4.24; p=0.010]. The results of the univariate analysis were summarized in Table 1. Apart from arterial hypertension, the previously mentioned factors were confirmed in a multivariable analysis. Urinary lithiasis was also found as a risk factor in the multivariate analysis (Table 2).
|
MDR prevalence |
p-value |
OR |
95% CI |
|
|
Arterial hypertension |
27.0% |
0.020 |
1.730 |
(1.089–2.748) |
|
Diabetes mellitus |
22.6% |
0.968 |
0.989 |
(0.577–1.694) |
|
Cardiovascular disease |
29.4% |
0.071 |
1.583 |
(0.960–2.612) |
|
Liver disease |
17.2% |
0.458 |
0.689 |
(0.256–1.854) |
|
Urinary lithiasis |
34.1% |
0.070 |
1.875 |
(0.942–3.732) |
|
Prior urinary tract infection |
40.6% |
0.013 |
2.509 |
(1.192–5.281) |
|
ASA III–IVa |
21.9% |
0.177 |
1.540 |
(0.820–2.891) |
|
Immunosupressionb |
37.5% |
0.010 |
2.254 |
(1.197–4.245) |
|
Urinary catheter prior admission |
26.3% |
0.205 |
1.345 |
(0.849–2.130) |
|
Urinary catheter during the hospitalization |
23.4% |
0.534 |
1.195 |
(0.681–2.097) |
aASA – Physical status classification according to the American Society of Anesthesiologists
bImmunosuppression includes patients with haematological neoplasms such as leukemia, lymphoma, multiple myeloma and those with acquired immune deficiency syndrome (AIDS) and treatment with immunosuppressive drugs.
|
|
p-value |
OR |
95% CI |
|
Arterial hypertension |
0.056 |
1.622 |
(0.987–2.665) |
|
Cardiovascular disease |
0.251 |
1.369 |
(0.801–2.339) |
|
Immunosupression |
0.023 |
2.133 |
(1.110–4.101) |
|
Urinary lithiasis |
0.018 |
2.390 |
(1.163–4.911) |
|
Prior urinary tract infection |
0.021 |
2.455 |
(1.145–5.261) |
MDRO were reported in 28.4% of patients where Enterobacteriaceae were isolated; 23.8% and 44.7% in those with E. coli (38 out of 160) and Klebsiella spp. (42 out of 94), respectively. No MDRO were reported in patients with isolation of Citrobacter spp. (0 out of 7) and Proteus spp. (0 out of 14). The percentages of MDRO in patients with Enterobacter spp., Morganella spp., and Serratia spp. isolation were 26.5% (9 out of 34), 21.4% (3 out of 14), and 50% (3 out of 6), respectively. Among cultures with the isolation of Enterobacteriaceae, 7% showed resistance to carbapenems. Two cases (1.3%) of E. coli resistant to carbapenems were reported. Among cultures with the isolation of Klebsiella spp., 10% were resistant to carbapenems. All carbapenemase-producing bacteria isolated from type OXA-48. Three out of 80 Enterococcus spp. were vancomycin-resistant. The rate of Pseudomonas aeruginosa resistant to at least three antibiotic groups was 36.3%. Table 3 reported the microbiological characteristics of the MDRO isolated. Cultures in which MDRO were isolated showed 75.8% and 64.8% susceptibility rates for carbapenems and amikacin, respectively. On the other hand, the cultures with the isolation of MDRO reported a resistance rate to fluoroquinolones of 84.2%. Finally, the empirical antibiotic treatment was not adequate in 28% of patients with MDRO in comparison with 13.2% of patients with HAIs due to non-MDRO.
| Number of MDRO | |
| Enterobacter spp. Carbapenem-resistant |
9 3 |
| Vancomycin-resistant Enterococcus spp. | 3 |
| Escherichia coli Carbapenem-resistant |
38 2 |
| Klebsiella spp. Carbapenem-resistant |
42 9 |
| Morganella spp. Carbapenem-resistant |
3 0 |
| Pseudomonas aeruginosa | 29 |
| Serratia marcescens Carbapenem-resistant |
3 0 |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 1 |
4 Discussion
One of the main consequences of antibiotics’ widespread use is the emergence of microorganisms with resistance to antibiotics. Nowadays, a primary concern for worldwide healthcare systems is the increasing prevalence of antibiotic resistance in HAIs and outpatient settings. Moreover, the emergence of MDRO is a worrisome point, as selecting an appropriate antibiotic treatment is a challenging task [11]. It is estimated that MDRO infections increase hospitalization costs by 10% to 30% [12], [13]. Our data showed that although continuous monitoring and prevention of infections has l0ed to a decrease in the global prevalence of HAI, there is an increasing prevalence of the presence of MDRO. Specifically, 22.8% were positive cultures with the isolation of MDRO, and up to 7% of patients with isolation of Enterobacteriaceae were producers of carbapenemases. Previous data from our center confirms that carbapenemase-producing Enterobacteriaceae infections represented 3.6% of all healthcare-associated infections and 9.7% of those caused by enterobacteria [14]. The outbreak of carbapenemase-producing bacteria is one of the most worrisome points, as in this case, the correct selection of antibiotics is a challenging task [1], [3], [14]. The situation is especially serious in some Asian countries, such as India and China, with very high rates of carbapenemase-producing MDRO [15], [16].
Nevertheless, identifying risk factors associated with the isolation of MDRO plays a fundamental role and may add in the choice of empirical antimicrobial therapy, especially if we suspect that we are facing an MDRO with resistance to carbapenems [14], [17]. According to our results, we confirm that the presence of immunosuppression, prior UTIs and urinary catheter in the upper urinary tract are risk factors for presenting MDRO infections. Those factors are also related to a higher risk of HAIs [2], [4], [18]. However, we did not identify, among the possible risk factors analyzed, that the presence of diabetes mellitus or high anaesthetic risk (ASA score III–IV) were predisposing factors for the isolation of MDRO. Among urological patients, those with a catheter in the upper urinary tract (ureteral double-J stent, nephrostomy tube, or percutaneous internal-external nephrostomy catheter) require special attention and show a high prevalence of infections due to MDRO [19], [20]. The risk of isolation of MDRO is especially high in those with a percutaneous internal-external nephrostomy catheter, up to 50% in our series.
One of the main issues when dealing with MDRO is the prescription of adequate empirical treatment [17], [21]. In our department, for those hospitalized in a urology ward with HAIs and risk factors for MDRO, the empirical treatment is based on carbapenems (the susceptibility rate was 63.8%) or aminoglycosides such as amikacin (the susceptibility rate was 66.3%). Aminoglycosides were the most common empirical treatment in penicillin allergic patients.
Hospital antibiotic stewardship programs and a continuous surveillance of infections are recommended in order to optimize the management of infections [22], [23], [24], [25]. Although the prevalence of MDRO is increasing globally, HAIs and MDRO infections must be evaluated locally and revised periodically in each center due to the variability of resistance with essential differences between continents, countries, and even centers in the same region [3], [26]. Therefore, based on clinical practice guidelines, each center has to collect its data on the prevalence of MDRO and design protocols for the prescription of empirical antibiotic therapy [11], [27], [28]. These measures are useful in order to optimize treatments and reduce complications, and will provide a better understanding of the development of resistance [13], [29]. Urologists must be aware that MDRO infections are common, and they are becoming increasingly aware. One of the main activities in urology to assess the characteristics of HAIs is the GPIU study [4], [5]. MDRO infections are a challenging task and may require management with the support of an infectious diseases specialist. However, as urologists manage the patients, they have to involve all diagnostic and therapeutic measures [30].
5 Conclusions
MDRO are more common in immunosuppressed patients, as well as those with previous UTIs, urinary catheter before admission, and urinary catheters into the upper urinary tract. Therefore, the prevalence of MDRO in urology is a problem of great importance, since up to 25% of the isolates showed MDRO. The evaluation of the prevalence of MDRO is the first step towards developing strategies aimed at better control of HAIs, in addition to knowing the risk factors predisposed to presenting MDRO. This information should be used to determine which antibiotic therapy is the most appropriate. Due to the variability in the prevalence of MDRO infections, multicenter and transnational studies are necessary to develop joint strategies as well as consensus to counteract the progression of resistance to antibiotic therapy.
Note
This chapter was first published in GMS Infectious Diseases [31].
Acknowledgments
We acknowledge the effort and collaboration of all personnel of the department of urology for preventing infections. Moreover, special thanks to all the residents of our department who have actively collected and reviewed the data.
Authors’ ORCIDs
- José Medina-Polo: 0000-0003-3626-8669
- Javier Gil-Moradillo: 0000-0002-8098-0218
- Alejandro González-Díaz: 0000-0002-3581-7200
- Pablo Abad-López: 0000-0001-7902-298X
- Rocío Santos-Pérez de la Blanca: 0000-0003-0063-6810
- Helena Peña-Vallejo: 0000-0001-6210-2318
- Julio Téigell-Tobar: 0000-0002-6873-8031
- Ángel Tejido-Sánchez: 0000-0001-8739-8370
Competing interests
The authors declare that they have no competing interests.
References
[1] Suarez C, Peña C, Arch O, Dominguez MA, Tubau F, Juan C, Gavaldá L, Sora M, Oliver A, Pujol M, Ariza J. A large sustained endemic outbreak of multiresistant Pseudomonas aeruginosa: a new epidemiological scenario for nosocomial acquisition. BMC Infect Dis. 2011 Oct;11:272. DOI: 10.1186/1471-2334-11-272[2] Medina-Polo J, Arrébola-Pajares A, Pérez-Cadavid S, Benítez-Sala R, Sopeña-Sutil R, Lara-Isla A, Alsonso-Isa M, Gil-Moradillo J, Justo-Quintas J, Miranda-Utrera N, Aguilar-Gisbert L, Passas-Martínez JB, Tejido-Sánchez Á. Extended-Spectrum Beta-Lactamase-Producing Bacteria in a Urology Ward: Epidemiology, Risk Factors and Antimicrobial Susceptibility Patterns. Urol Int. 2015;95(3):288-92. DOI: 10.1159/000439441
[3] Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016 Nov;37(11):1288-1301. DOI: 10.1017/ice.2016.174
[4] Tandogdu Z, Bartoletti R, Cai T, Çek M, Grabe M, Kulchavenya E, Köves B, Menon V, Naber K, Perepanova T, Tenke P, Wullt B, Johansen TE, Wagenlehner F. Antimicrobial resistance in urosepsis: outcomes from the multinational, multicenter global prevalence of infections in urology (GPIU) study 2003–2013. World J Urol. 2016 Aug;34(8):1193-200. DOI: 10.1007/s00345-015-1722-1
[5] Tandogdu Z, Cek M, Wagenlehner F, Naber K, Tenke P, van Ostrum E, Johansen TB. Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J Urol. 2014 Jun;32(3):791-801. DOI: 10.1007/s00345-013-1154-8
[6] Pitout JD. Enterobacteriaceae that produce extended-spectrum β-lactamases and AmpC β-lactamases in the community: the tip of the iceberg? Curr Pharm Des. 2013;19(2):257-63.
[7] Hayami H, Takahashi S, Ishikawa K, Yasuda M, Yamamoto S, Wada K, Kobayashi K, Hamasuna R, Minamitani S, Matsumoto T, Kiyota H, Tateda K, Sato J, Hanaki H, Masumori N, Nishiyama H, Miyazaki J, Fujimoto K, Tanaka K, Uehara S, Matsubara A, Ito K, Hayashi K, Kurimura Y, Ito S, Takeuchi T, Narita H, Izumitani M, Nishimura H, Kawahara M, Hara M, Hosobe T, Takashima K, Chokyu H, Matsumura M, Ihara H, Uno S, Monden K, Sumii T, Kawai S, Kariya S, Sato T, Yoshioka M, Kadena H, Matsushita S, Nishi S, Hosokawa Y, Shirane T, Yoh M, Watanabe S, Makinose S, Uemura T, Goto H. Second nationwide surveillance of bacterial pathogens in patients with acute uncomplicated cystitis conducted by Japanese Surveillance Committee from 2015 to 2016: antimicrobial susceptibility of Escherichia coli, Klebsiella pneumoni
[8] Johansen TE, Cek M, Naber KG, Stratchounski L, Svendsen MV, Tenke P; PEP and PEAP-study investigators; Board of the European Society of Infections in Urology. Hospital acquired urinary tract infections in urology departments: pathogens, susceptibility and use of antibiotics. Data from the PEP and PEAP-studies. Int J Antimicrob Agents. 2006 Aug;28 Suppl 1:S91-107. DOI: 10.1016/j.ijantimicag.2006.05.005
[9] Jiménez-Alcaide E, Medina-Polo J, García-González L, Arrébola-Pajares A, Guerrero-Ramos F, Pérez-Cadavid S, Sopeña-Sutil R, Benítez-Sala R, Alonso-Isa M, Lara-Isla A, Passas-Martínez JB, Tejido-Sánchez Á. Infecciones del tracto urinario de origen hospitalario en pacientes portadores de catéter urinario: factores de riesgo, características microbiológicas y resistencias a antibióticos [Healthcare-associated urinary tract infections in patients with a urinary catheter: Risk factors, microbiological characteristics and patterns of antibiotic resistance]. Arch Esp Urol. 2015 Jul-Aug;68(6):541-50.
[10] Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268-81. DOI: 10.1111/j.1469-0691.2011.03570.x
[11] Wagenlehner FM, Naber KG. Emergence of antibiotic resistance and prudent use of antibiotic therapy in nosocomially acquired urinary tract infections. Int J Antimicrob Agents. 2004 Mar;23 Suppl 1:S24-9. DOI: 10.1016/j.ijantimicag.2003.09.004
[12] Tenke P, Kovacs B, Bjerklund Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008 Feb;31 Suppl 1:S68-78. DOI: 10.1016/j.ijantimicag.2007.07.033
[13] Hedrick TL, Sawyer RG. Health-care-associated infections and prevention. Surg Clin North Am. 2005 Dec;85(6):1137-52, ix. DOI: 10.1016/j.suc.2005.09.013
[14] Justo-Quintas J, Medina-Polo J, Gil-Moradillo J, Jaén-Herreros F, Lara-Isla A, Tejido-Sánchez Á. Infections by carbapenemase-producing enterobacteriaceae in a department of urology. A new challenge. Actas Urol Esp (Engl Ed). 2018 Apr;42(3):170-5. DOI: 10.1016/j.acuro.2017.08.004
[15] Pragasam AK, Veeraraghavan B, Nalini E, Anandan S, Kaye KS. An update on antimicrobial resistance and the role of newer antimicrobial agents for Pseudomonas aeruginosa. Indian J Med Microbiol. 2018 Jul-Sep;36(3):303-16. DOI: 10.4103/ijmm.IJMM_18_334
[16] Peng Y, Bi J, Shi J, Li Y, Ye X, Chen X, Yao Z. Multidrug-resistant Pseudomonas aeruginosa infections pose growing threat to health care-associated infection control in the hospitals of Southern China: a case-control surveillance study. Am J Infect Control. 2014 Dec;42(12):1308-11. DOI: 10.1016/j.ajic.2014.08.006
[17] Wagenlehner FM, Weidner W, Naber KG. Pharmacokinetic characteristics of antimicrobials and optimal treatment of urosepsis. Clin Pharmacokinet. 2007;46(4):291-305. DOI: 10.2165/00003088-200746040-00003
[18] Medina-Polo J, Sopeña-Sutil R, Benítez-Sala R, Lara-Isla A, Alonso-Isa M, Gil-Moradillo J, Justo-Quintas J, García-Rojo E, González-Padilla DA, Passas-Martínez JB, Tejido-Sánchez Á. Prospective study analyzing risk factors and characteristics of healthcare-associated infections in a Urology ward. Investig Clin Urol. 2017 Jan;58(1):61-9. DOI: 10.4111/icu.2017.58.1.61
[19] Lara-Isla A, Medina-Polo J, Alonso-Isa M, Benítez-Sala R, Sopeña-Sutil R, Justo-Quintas J, Gil-Moradillo J, González-Padilla DA, García-Rojo E, Passas-Martínez JB, Tejido-Sánchez Á. Urinary Infections in Patients with Catheters in the Upper Urinary Tract: Microbiological Study. Urol Int. 2017;98(4):442-8. DOI: 10.1159/000467398
[20] Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006 Sep;16(9):2016-30. DOI: 10.1007/s00330-005-0136-7
[21] Gross PA. Hypotension and mortality in septic shock: the “golden hour”. Crit Care Med. 2006 Jun;34(6):1819-20. DOI: 10.1097/01.CCM.0000220054.95214.7D
[22] Medina-Polo J, Gil-Moradillo J, Justo-Quintas J, González-Padilla DA, García-Rojo E, González-Díaz A, Abad-López P, Hernández-Arroyo M, Santos-Pérez de la Blanca R, Peña-Vallejo H, Téigell-Tobar J, López-Medrano F, Tejido-Sánchez Á. Prevention of healthcare-associated infections (HAIs) in a surgical urology ward: observational study-analysis of the problem and strategies for implementation. World J Urol. 2020 Jan;38(1):3-8. DOI: 10.1007/s00345-019-02648-3
[23] Doyle B, Mawji Z, Horgan M, Stillman P, Rinehart A, Bailey J, Mullin E Jr. Decreasing nosocomial urinary tract infection in a large academic community hospital. Lippincotts Case Manag. 2001 May-Jun;6(3):127-36. DOI: 10.1097/00129234-200105000-00007
[24] Spoorenberg V, Hulscher ME, Akkermans RP, Prins JM, Geerlings SE. Appropriate antibiotic use for patients with urinary tract infections reduces length of hospital stay. Clin Infect Dis. 2014 Jan;58(2):164-9. DOI: 10.1093/cid/cit688
[25] Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, Hooton TM. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985 Feb;121(2):182-205. DOI: 10.1093/oxfordjournals.aje.a113990
[26] Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK; National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008 Nov;29(11):996-1011. DOI: 10.1086/591861
[27] Wagenlehner FM, Naber KG. Treatment of bacterial urinary tract infections: presence and future. Eur Urol. 2006 Feb;49(2):235-44. DOI: 10.1016/j.eururo.2005.12.017
[28] Bonkat G, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Köves B, Schubert S, Wagenlehner F, Devlies W, Horváth J, Mantica G, Mezei T, Pilatz A, Pradere B, Veeratterapillay R; European Association of Urology (EAU). Guidelines on Urological Infections. Arnhem: Uroweb EAU; 2020 [cited 2021 Feb 21]. Available from: http://uroweb.org/guideline/urological-infections/
[29] Johansen TE. Nosocomially acquired urinary tract infections in urology departments. Why an international prevalence study is needed in urology. Int J Antimicrob Agents. 2004 Mar;23 Suppl 1:S30-4. DOI: 10.1016/j.ijantimicag.2003.09.003
[30] Lebentrau S, Gilfrich C, Vetterlein MW, Schumacher H, Spachmann PJ, Brookman-May SD, Fritsche HM, Schostak M, Wagenlehner FM, Burger M, May M; MR2 study group. Impact of the medical specialty on knowledge regarding multidrug-resistant organisms and strategies toward antimicrobial stewardship. Int Urol Nephrol. 2017 Aug;49(8):1311-8. DOI: 10.1007/s11255-017-1603-1
[31] Medina-Polo J, Gil-Moradillo J, González-Díaz A, Abad-López P, Santos-Pérez de la Blanca R, Hernández-Arroyo M, Peña-Vallejo H, Téigell-Tobar J, Calzas-Montalvo C, Caro-González P, Miranda-Utrera N, Tejido-Sánchez Á. Observational study over 8-year period evaluating microbiological characteristics and risk factor for isolation of multidrug-resistant organisms (MDRO) in patients with healthcare-associated infections (HAIs) hospitalized in a urology ward. GMS Infect Dis. 2021;9:Doc04. DOI: 10.3205/id000073




