Body temperature and thermal imaging
Malcolm Mitchell 2
Björn Kuhla 3
Sadjad Danesh Mesgaran 3
1 Teagasc, Animal and Grassland Research and Innovation Centre, Fermoy, Irland
2 Scotland's Rural College, Edinburgh, United Kingdom
3 Leibniz Institute for Farm Animal Biology (FBN), Dummerstorf, Deutschland
Introduction
Body temperature data is often used in cattle to assess different reproductive events such as oestrus [1] and pregnancy [2] or to identify prevalence of various infectious diseases [3]. Therefore, recording body temperature is important when considering both the health and reproductive status of livestock. In addition, when body temperature is not affected by physical activity, it can be a good indicator of heat stress in cows [4]. Body temperature is often measured during experiments with cattle, yet there is a lack of introduction and development of tools to measure this parameter consistently and precisely [5]. Core body temperature measurements indicate the internal temperature of body, in close proximity to the main organs e.g., heart, brain or viscera. It can be evaluated by rectal, vaginal, tympanic, vascular, intra-peritoneal, or digestive tract sensors [6]. Other temperature measurements such as mid-peripheral and peripheral (from animal's skin, coat, udder, etc) can also be acquired, but are further from the body's core and will deviate more. Rectal temperature is widely considered as the ‘gold standard’ way to measure core body temperature, and can easily be measured using various handheld thermometers. In addition to this, scientists have developed devices (probes) in order to constantly monitor rectal temperature thereby avoiding labour-intensive handheld measurement [7] and handling of animals. Recent work has shown that rectal and tympanic temperatures are more reliable than subdermal temperatures under various environmental conditions [8]. Vaginal temperature loggers are also available and provide data sets in the absence of personnel, thereby reducing any unwanted behavioural and endocrinological reactions of cattle to human presence [9], [10].
Infrared thermography has been used by practitioners and scientists to study different physiological aspects of animals [11]. This technique allows researchers to conduct measurements of the animal at close range (<1 m) or at large distances (>1,000 m) without disrupting the animal's normal behaviour. Thermal imaging is a non-intrusive and cost-effective method, based upon the Stefan-Boltzmann law, i.e. objects emit energy proportional to their temperature. Thermal cameras have the ability to capture the emitted infrared radiation and display it as a pictorial representation – a thermogram – of the examined object's surface temperature [12], [13]. Dependent on the colour scale chosen/available on the device, the warmest areas are generally depicted as white or red, while the coolest areas appear blue or black [14]. Thermal cameras have been used to monitor in different physiological aspects of livestock, particularly cattle. In terms of cattle welfare, thermal imaging has been engaged to detect pain, associated with routine husbandry practices such as tail docking, catheterisation, ear implantation [15], [16], [17]. Thermal imaging has shown to be a promising tool for early diagnosis of bovine viral diarrhoea infections or bovine respiratory diseases in dairy and beef calves, identifying potential thermal changes after facial scanning [18], [19]. Detection of mastitis and lameness has been the focus of much of the thermal imaging research done in adult cows. Monitoring changes in udder skin surface temperature with thermal cameras has been used to screen for subclinical mastitis in cattle and has been shown to have a high predictive diagnostic ability, comparable to that of reference mastitis tests [20]. Hovinen [21] showed that thermal cameras were able to detect temperature rises from 1 to 1.5 degrees in left forequarters of udders with artificially induced clinical mastitis. Despite this, the accuracy of this technique needs further validation in cattle under different environmental conditions. Alsaaod and Büscher [22] sought to investigate claw pathology via thermography to diagnose lameness in cattle. They successfully measured higher surface temperatures in lame limbs, which were the result of hoof lesion. Others have also diagnosed lameness in cattle by thermal scanning of the animal's hoof [23], [24]. Thermal imaging is an interesting tool for the early detection of inflammation associated with lameness in cattle, but it has been noted that further validation under different environmental condition is required.
Body temperature
Prerequisites
This guideline provides information on performing rectal and vaginal temperature measurement in cattle. This guideline presumes that measurement of the rectal temperature is carried out manually via a digital thermometer in cattle. The guideline also presumes that vaginal temperature will be conducted via microprocessor-controlled data loggers (vaginal loggers). The Animal Trait Ontology for Livestock (ATOL) numbers linked with this guideline are: ATOL_0000281, ATOL_0000332, ATOL_0000329 and ATOL_0000335 (for the complete list of ATOL, please visit https://www.atol-ontology.com/en/erter-2/).
A – Assessing rectal temperature during experimental trials
- Rectal temperature may be taken to confirm sickness or oestrus or during hot weather periods.
- A fever should be defined as when the rectal temperature exceeds 39–39.3°C. Immediate veterinary treatment should be sought where the rectal temperature exceeds 39.5°C.
- The rectal temperature of cattle 8–10 d postpartum should be taken to monitor the health of the animal post calving. Particularly during the first 3 d post calving, rectal temperature must be determined 2–3 times per d.
- Prior to use, the accuracy of the digital thermometer should be evaluated using a water bath with a calibrated liquid-in-glass thermometer. Details of the procedure has been elaborately described by Burfeind [25].
- The accuracy of the digital thermometer should be periodically checked (every 4–6 months) following the procedure stated in point 4 (part A).
- If different types/models of thermometers are used in the unit, variation in recordings between the instruments should be carefully determined.
- The thermometer must have contact with the rectal mucosa for accurate temperature readings. Details of insertion depth with different thermometers are outlined in point 8 (part A).
- Digital thermometers with angled probes must be inserted in a way where the entire angled probe disappears into the anus. Thermometers with a straight probe should be inserted in the rectum up to the edge of the display. Regardless of the thermometer probe type, penetration depth must be consistent between 2 or more measurements.
- The thermometer has to be held in place in the rectum for a minimum 1–2 min, prior to taking out and reading.
- After removing the thermometer, faecal material and mucus should be removed from it and it should be thoroughly cleaned and disinfected using 70% ethanol solution.
- If an animal defecates during rectal temperature collection, this should be recorded and care taken in the final analysis. Rectal temperature may notably increase during and shortly after defecation.
B – Consecutive measuring of core body temperature with vaginal probes
- Before use, the accuracy of the vaginal data logger should be tested with a calibrated thermometer (as gold standard). Both the data logger and thermometer should be placed in the same water bath with a gradual increase in temperature from 35 to 40°C and paired readings recorded for comparison [26].
- To precisely evaluate vaginal data loggers in the field, rectal temperature should be examined daily, with a minimum of 6–8 times as a reference. Data from the vaginal loggers (as 1–2 h intervals) should then be compared with the corresponding rectal temperature.
- The logger should be encapsulated within a plastic cover prior to insertion in the vagina. This cover allows the logger to be held within the animal's vagina. It is also possible to attach the temperature logger to a progesterone-free, modified vaginal controlled internal drug release system.
- The data logger has to be positioned in close proximity to the cervix for accurate data acquisition.
- The insertion of the data logger into the vagina should be performed by an experienced veterinarian/operator.
- The insertion depth of the logger probes should be minimum 10 cm into vaginal cavity.
- Vaginal temperature loggers can be used from 24 hours up to 3 weeks (dependent on the manufacturer). However, for measuring periods >4 d, the orientation of the loggers should be checked every 48–72 h via rectal palpation.
- The animal must be monitored during vaginal temperature recording for any signs of infection or discomfort due to the logger insertion.
- The vaginal logger along with its cover should be thoroughly wiped and disinfected after the measurement is complete.
- The operator must be aware of generated artefact measurements from the system due to logger movements, or influx of ambient air, and should omit those from final analysis.
Thermal imaging
Prerequisites
This section focuses on conducting thermal imaging of cattle using thermal cameras. The Animal Trait Ontology (ATOL) and Environment Ontology (EOL) for Livestock numbers linked with this guideline are: ATOL_0001491, ATOL_0001393, EOL_0000008 and EOL_0000178 (for the complete list of ATOL, please visit https://www.atol-ontology.com/en/erter-2/).
A – General good practice for thermal imaging
- Staff members have to be aware that a substantial amount of time and practice is required to familiarise themselves with using the thermal camera; focusing successfully or navigating through the settings. Hence, a significant amount of practicing is required prior to the actual trial.
- As focus is vital for thermal images, the operator should use a tripod whenever possible to enhance the focus.
- Standard protocol criteria such as a) distance, b) angle, and c) orientation (left, right, underneath) to the object should be established for the experiment. All staff members, personnel or students must then carry out the imaging using those specific criteria.
- All environmental conditions have to be measured regularly, and camera settings or object parameters should be regulated when the temperature and humidity changes by more than 2–3 degrees and 10%, respectively.
- The operator must keep accurate records of animal number/ID, image number, the time that image were taken and the content of the image. Any issues with, or dirt on the animal that could affect the image, should be noted.
- During the imaging process, it is important to keep the animal away from direct sunlight, strong reflections, drafts or wetness (saliva, urine, faeces, rain and snow). In particular, the use of warm or hot water must be completely avoided as this will render the image useless.
- The operator should not touch the area that the imaging will be conducted on as this leads to transference of heat. If it is not possible to avoid touching, then the operator must wear gloves.
- The area where imaging takes place should be kept consistent, i.e. if it is dry, then all animals should remain dry.
- The operator should not drop the camera or touch the front of the lens.
- The laser of the camera should not be pointed directly at the face or eyes of the animal or any human.
B – Imaging with thermal cameras
- Generally, wide angle lenses are required for imaging the cows/heifers. During the attachment of lenses, the operator should avoid touching any part of the back or front of the lens itself.
- When a battery change is required, the operator should ensure to properly engage the new battery.
- The operator can navigate through settings from the LCD screen of the camera or through the viewfinder. However, it is advisable that the operator uses the viewfinder in bright conditions to ensure the view is not obscured by light.
- The operator should check all settings, especially the environmental conditions, and input those into the object parameters. Parameters used must correctly reflect where the imaging will take place.
- The reflected temperature should be tested and changed, based upon the environment of imaging. The reflective temperature allows the operator to compensate for temperatures from surrounding objects on the target object. If a reflection of thermal energy from the surrounding objects is suspected, the camera should be moved around in the area surrounding the target, whilst checking if the hot or cold spot moves with the camera. If the hot or cold spot moves with the camera, it is a reflection from another object; if does not, it is a true hot or cold spot.
- After setting the basic parameters, the camera should be used in the auto mode, which will eventually change the temperature level and colours as conditions changes.
- If automatic function does not fit the environmental conditions of the unit, then manual adjustments can be made using the level and options of the camera.
- Staff members should make shure that the date and time settings of the camera are correct to allow images to be associated with animals accurately, particularly after any summer or winter clock changes.
- The operator should ensure that the focus of the camera is as sharp as possible prior saving the image. The focus/zoom button can help the operator; however, the focus ring on the camera lens should be used to sharpen the image. Out-of-focus images will be hard to analyse, and therefore practice is required, particularly with moving objects.
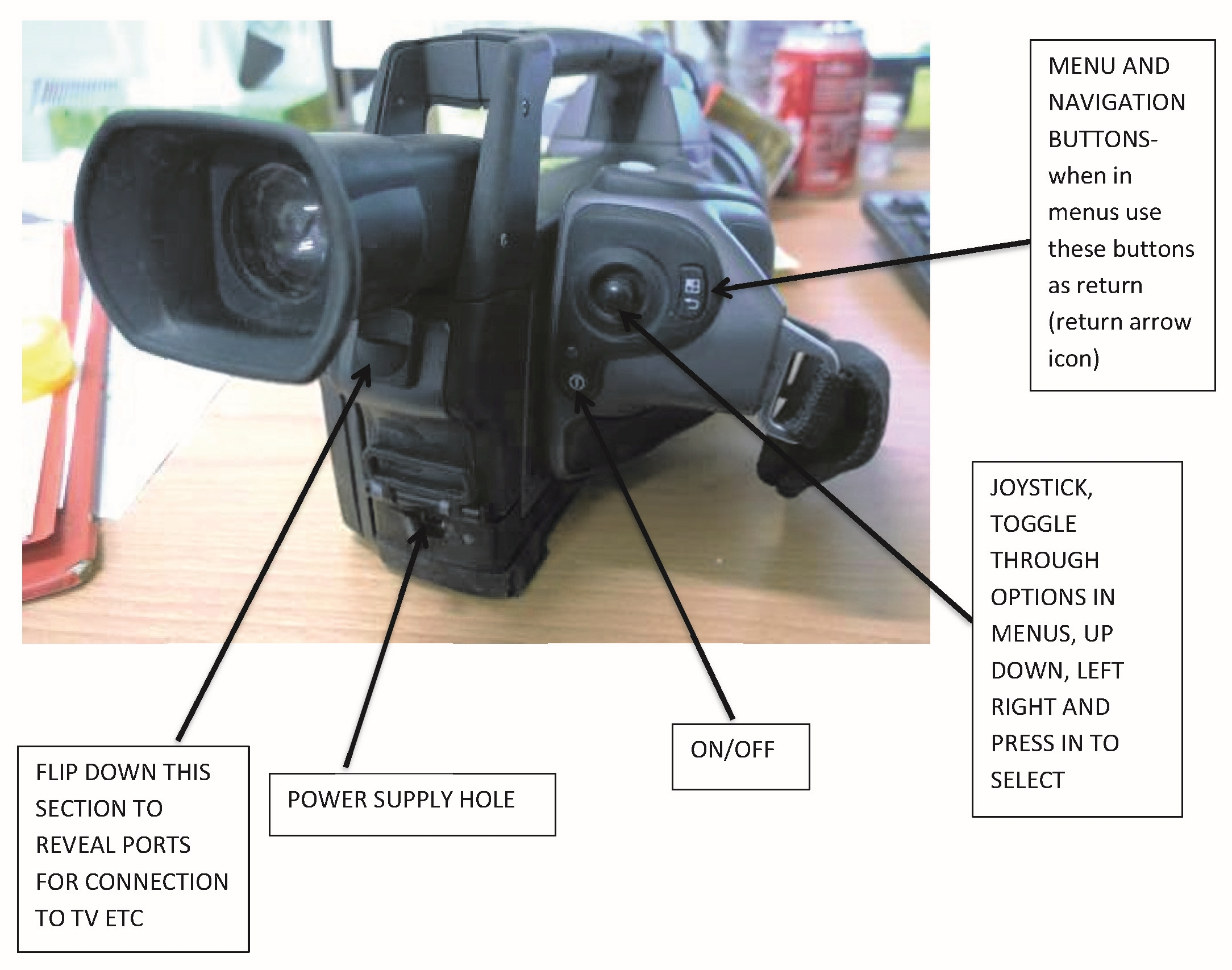
References
[1] Talukder S, Kerrisk KL, Ingenhoff L, Thomson PC, Garcia SC, Celi P. Infrared technology for estrus detection and as a predictor of time of ovulation in dairy cows in a pasture-based system. Theriogenology. 2014;81(7):925-35. DOI: 10.1016/j.theriogenology.2014.01.009[2] Gil Z, Kural J, Szarek J, Wierzchos E. Increase in milk and body temperature of cows as a sign of embryo entry into the uterus. Theriogenology. 2001;56(4):685-97. DOI: 10.1016/s0093-691x(01)00600-8
[3] Schaefer AL, Cook NJ, Bench C, Chabot JB, Colyn J, Liu T, et al. The non-invasive and automated detection of bovine respiratory disease onset in receiver calves using infrared thermography. Res Vet Sci. 2012;93(2):928-35. DOI: 10.1016/j.rvsc.2011.09.021
[4] Mader TL, Gaughan JB, Johnson LJ, Hahn GL. Tympanic temperature in confined beef cattle exposed to excessive heat load. Int J Biometeorol. 2010;54:629-35. DOI: 10.1007/s00484-009-0229-0
[5] Hahn GL, Chen YR, Nienaber JA, Eigenberg RA, Parkhurst AM. Characterizing animal stress through fractal analysis of thermoregulatory responses. J Therm Biol. 1992;17(2):115-20. DOI: 10.1016/0306-4565(92)90008-4
[6] Sellier N, Guettier E, Staub C. A review of methods to measure animal body temperature in precision farming. J Agric Sci Technol. 2014;2:74-99. DOI: 10.7726/ajast.2014.1008
[7] Reuter RR, Carroll JA, Hulbert LE, Dailey JW, Galyean ML. Technical note: Development of a self-contained, indwelling rectal temperature probe for cattle research. J Anim Sci. 2010;88(10):3291-5. DOI: 10.2527/jas.2010-3093
[8] Carroll JA, Reuter RR, Chase CC Jr, Coleman SW, Riley DG, Spiers DE, et al. Profile of the bovine acute-phase response following an intravenous bolus-dose lipopolysaccharide challenge. Innate Immun. 2009;15(2):81-9. DOI: 10.1177/1753425908099170
[9] Curley KO Jr, Neuendorff DA, Lewis AW, Cleere JJ, Welsh TH Jr, Randel RD. Functional characteristics of the bovine hypothalamic-pituitary-adrenal axis vary with temperament. Horm Behav. 2008;53(1):20-7. DOI: 10.1016/j.yhbeh.2007.08.005
[10] Burdick NC, Carroll JA, Hulbert LE, Dailey JW, Ballou MA, Randel RD, et al. Temperament influences endotoxin-induced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 2011;17(4):355-64. DOI: 10.1177/1753425910379144
[11] McCafferty DJ. The value of infrared thermography for research on mammals: previous applications and future directions. Mamm Rev. 2007;37(3):207-23. DOI: 10.1111/j.1365-2907.2007.00111.x
[12] Eddy AL, Van Hoogmoed LM, Snyder JR. The role of thermography in the management of equine lameness. Vet J. 2001;162(3):172-81. DOI: 10.1053/tvjl.2001.0618
[13] Alsaaod M, Schaefer AL, Büscher W, Steiner A. The Role of Infrared Thermography as a Non-Invasive Tool for the Detection of Lameness in Cattle. Sensors. 2015;15(6):14513-25. DOI: 10.3390/s150614513
[14] Colak A, Polat B, Okumus Z, Kaya M, Yanmaz LE, Hayirli A. Short communication: early detection of mastitis using infrared thermography in dairy cows. J Dairy Sci. 2008;91(11):4244-8. DOI: 10.3168/jds.2008-1258
[15] Eicher SD, Cheng HW, Sorrells AD, Schutz MM. Short Communication: Behavioral and Physiological Indicators of Sensitivity or Chronic Pain Following Tail Docking1. J Dairy Sci. 2006;89(8):3047-51. DOI: 10.3168/jds.S0022-0302(06)72578-4
[16] Stewart M, Webster JR, Verkerk GA, Schaefer AL, Colyn JJ, Stafford KJ. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol Behav. 2007;92(3):520-5. DOI: 10.1016/j.physbeh.2007.04.034
[17] Schwartzkopf-Genswein KS, Stookey JM. The use of infrared thermography to assess inflammation associated with hot-iron and freeze branding in cattle. Can J Anim Sci. 1997;77(4):577-83. DOI: 10.4141/A97-019
[18] Schaefer AL, Cook N, Tessaro SV, Deregt D, Desroches G, Dubeski PL, et al. Early detection and prediction of infection using infrared thermography. Can J Anim Sci. 2004;84(1):73-80. DOI: 10.4141/A02-104
[19] Schaefer AL, Cook NJ, Church JS, Basarab J, Perry B, Miller C, et al. The use of infrared thermography as an early indicator of bovine respiratory disease complex in calves. Res Vet Sci. 2007;83(3):376-84. DOI: 10.1016/j.rvsc.2007.01.008
[20] Polat B, Colak A, Cengiz M, Yanmaz LE, Oral H, Bastan A, et al. Sensitivity and specificity of infrared thermography in detection of subclinical mastitis in dairy cows. J Dairy Sci. 2010;93(8):3525-32. DOI: 10.3168/jds.2009-2807
[21] Hovinen M, Siivonen J, Taponen S, Hänninen L, Pastell M, Aisla AM, et al. Detection of clinical mastitis with the help of a thermal camera. J Dairy Sci. 2008;91(12):4592-8. DOI: 10.3168/jds.2008-1218
[22] Alsaaod M, Büscher W. Detection of hoof lesions using digital infrared thermography in dairy cows. J Dairy Sci. 2012;95(2):735-42. DOI: 10.3168/jds.2011-4762
[23] Nikkhah A, Plaizier JC, Einarson MS, Berry RJ, Scott SL, Kennedy AD. Short Communication: Infrared Thermography and Visual Examination of Hooves of Dairy Cows in Two Stages of Lactation. J Dairy Sci. 2005;88(8):2749-53. DOI: 10.3168/jds.S0022-0302(05)72954-4
[24] Harris-Bridge G, Young L, Handel I, Farish M, Mason C, Mitchell MA, et al. The use of infrared thermography for detecting digital dermatitis in dairy cattle: What is the best measure of temperature and foot location to use? Vet J. 2018;237:26-33. DOI: 10.1016/j.tvjl.2018.05.008
[25] Burfeind O, von Keyserlingk MAG, Weary DM, Veira DM, Heuwieser W. Short communication: Repeatability of measures of rectal temperature in dairy cows. J Dairy Sci. 2010;93(2):624-7. DOI: 10.3168/jds.2009-2689
[26] Vickers LA, Burfeind O, von Keyserlingk MAG, Veira DM, Weary DM, Heuwieser W. Technical note: Comparison of rectal and vaginal temperatures in lactating dairy cows. J Dairy Sci. 2010;93(11):5246-51. DOI: 10.3168/jds.2010-3388




