Dupuytren’s Disease – the role of hand therapy
Catherine Ball 2
1 Department of Clinical Sciences/College of Health and Life Sciences, Brunel University London, Uxbridge, United Kingdom
2 Kennedy Institute of Rheumatology, University of Oxford, Headington, United Kingdom
Abstract
This chapter will provide an overview of the principles of hand therapy intervention used in the treatment of Dupuytren’s Disease and the evidence to support it.
Early disease and impact on hand function is discussed and the rehabilitation process is presented sequentially. It is not intended as a manual to treat Dupuytren’s Disease. The authors will present usual practice, evidence where available and highlight their preferred treatment. The current topical debate regarding the use of splinting is reported.
Introduction to therapy and aims of chapter
A hand therapist is an occupational therapist or physiotherapist who specialises in the rehabilitation of patients with conditions affecting the hands and upper limb. Rehabilitation aims to facilitate the return of functional performance to the individual’s hand [1], [2].
Whilst there is a body of evidence evaluating surgery in combination with hand therapy, there is limited data demonstrating the benefit of hand therapy as an independent treatment. Most treatment is based on general principles of hand rehabilitation following trauma or medical intervention. Principles that come under this heading include oedema and scar management, where it is accepted practice that intervention is required to improve hand function. There has been some limited research into the use of exercise and grip strengthening in patients with Dupuytren’s Disease whilst a growing debate regarding the indications for splinting has prompted research into its use.
Early disease and conservative treatment
Patients seek medical opinion and ultimately medical intervention following recognition or acknowledgement that the disease progression has begun to affect their functional ability of their hand [3]. As a result, in practice hand therapists do not often receive referrals for patients with early disease.
The use of hand therapy modalities: ultrasound [4], splinting [5], [6] , massage and exercises [7] as an alternative to corrective procedures has been investigated in maintaining finger extension in early Dupuytren’s Disease. However there is insufficient evidence to definitively support, or reject, their use either as an alternative treatment or prophylactically in early disease. In practice splinting is an option that may be considered for patients choosing to delay a corrective procedure for personal reasons, as a pre-operative measure while waiting for further treatment or for those unable to undergo a corrective procedure. It may also be the patient’s treatment of choice to maintain their current level of function [5], [6].
Impact on function
The location and severity of the Dupuytren’s involvement will greatly affect the functional impact [8]. In practice patients report a contracture of the proximal interphalangeal (PIP) joint inhibiting function to a greater extent than the metacarpophalangeal (MCP) [9]. Restriction of the PIP joint extension reduces the contact area of the palm when grasping, for example, when holding a mug. Reduced extension also makes the finger more liable to injury [3]. Additionally, PIP joint involvement may be more noticeable to others in social situations, for example receiving items from others and shaking hands [1], [3]. There is potential for the patient to compensate for the PIP joint deformity by hyperextending the MCP joint [8] which can ultimately lead to the development of a secondary deformity involving the distal interphalangeal (DIP) joint. The DIP becomes hyperextended resulting from the altered alignment of the extensor tendon mechanism within the PIP joint. In time this leads to the reduction of the ability to flex at the DIP joint. Although hand therapists infrequently have the luxury to recommend rehabilitation preoperatively, the authors advocate the importance of providing advice to patients before the corrective procedure in order to actively maintain, or improve, DIP flexion through specific exercises as seen in figure 1, thereby maximising the potential gain [10].
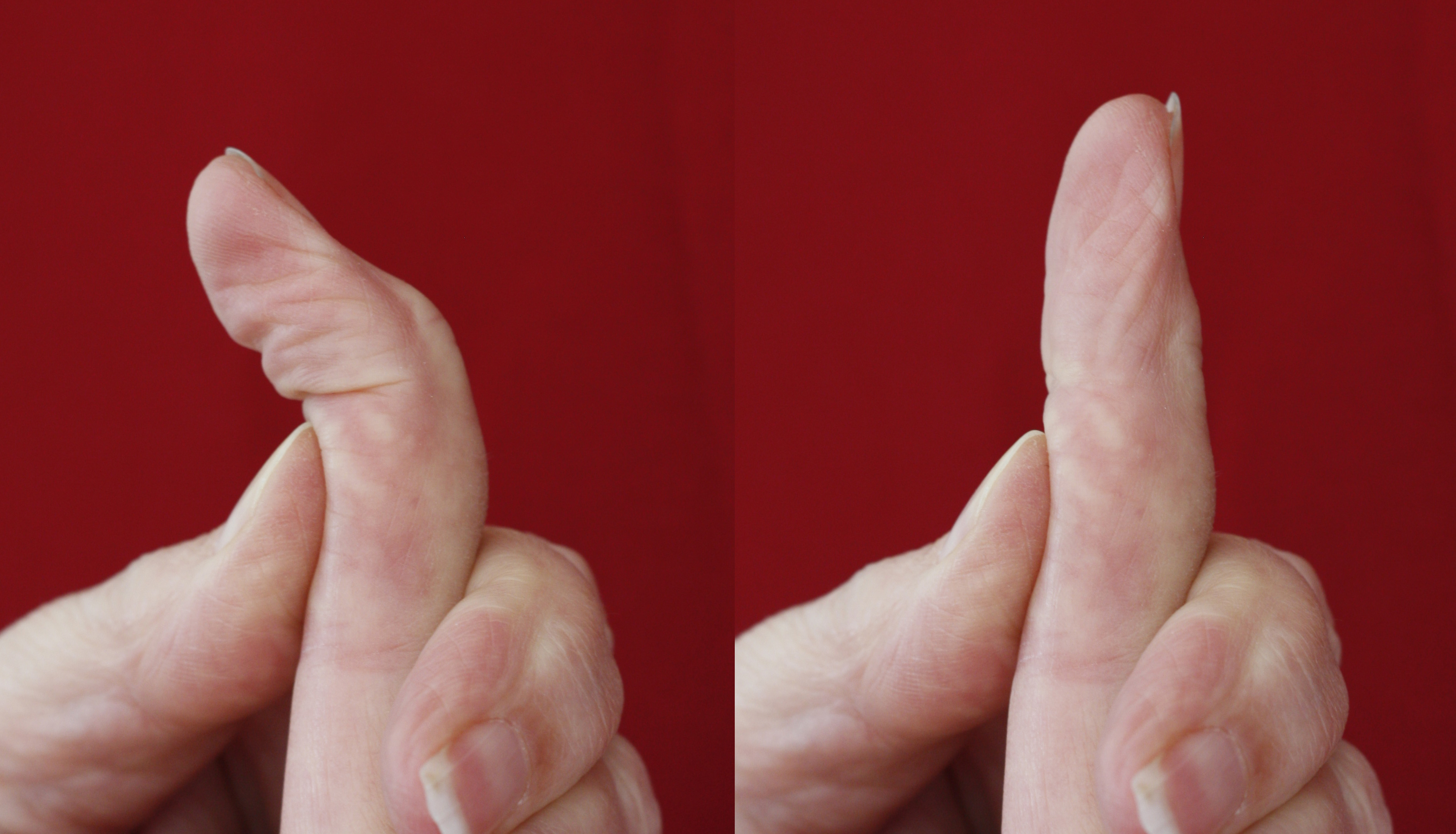
Prior to corrective procedure
The effect of the disease severity and the patient’s ability to use their hand puts the rehabilitation into context [8], [11]. The recording of physical and hand function measures do not only allow the final outcome to be quantified but enables the multi-disciplinary team to realistically tailor treatment according to the individual’s specific needs [1].
A careful selection of assessments is required to best reflect function, record progress and eventual outcomes. It is therefore recommended that assessments should include a combination of Patient Reported Outcome Measures (PROMS), Patient Reported Experience Measure (PREMS) and physical measures that can be reliably repeated post operatively [12].
Hand rehabilitation
Corrective procedures range from extensive surgery, for example fasciectomy and skin grafting, to less invasive procedures such as needle fasciotomy and enzymatic fasciotomy. Following any hand procedure, hand function will be compromised and rehabilitation may be required [13], [14]. Referral to hand therapy should be considered for all patients who have undergone any corrective procedure, especially those involving the PIP joint, so they can be treated according to need. In some units the hand therapist carries out the second stage of the enzymatic fasciotomy before rehabilitation commences.
The extent of post-operative rehabilitation will vary depending upon the outcome gained from the corrective procedure. Therapists are encouraged to tailor the treatment according to clinical and the individual’s need rather than protocol led.
After a detailed hand assessment a variety of modalities may be used to:
- Reduce post-operative oedema
- Increase range of motion (ROM)
- Improve pain free function (patient specified) and grip
- Minimise scar formation
Rehabilitation usually commences with gentle functional use when wound healing takes place. Dressings are reduced 3–7 days after surgery, usually by the hand therapist and rehabilitation commenced [1], [11], [15], [16].
Immediately following corrective procedure
Immediately following the corrective procedure it is common practice to advise the patient to elevate the arm and mobilise the digits within pain limits to reduce oedema. Care should be taken to not compromise wound healing thus maximising the rehabilitation given thereafter. Reducing oedema also limits the likelihood of adhesion formation and its respective complications which can inhibit movement [17].
On reduction of dressings
Movement and function
A more structured exercise programme is given to further increase active ROM including individual and combined joint exercises [1], [18]. Gentle passive exercises can be carried out in combination with active exercises without compromising wound healing [19] and can be increased over time. This is complemented with a home exercise programme for the patient to carry out between scheduled appointments [20], [21] which should include graded exercises [22]. Following the corrective procedure there is a need to increase extension but this should not be at the expense of flexion [23]. The return to patient’s usual function is increased during the rehabilitation period commencing with light activities [1], for example, fastening buttons, using a phone, writing and keyboard use.
Splints/orthotics
Historically splints have been used to maintain and/or improve extension gained from the corrective intervention [24], [25]. Static splinting is usually prescribed for night use thus limiting the impact on function during the day [10], [26]. A splint is fabricated by the hand therapist using thermoplastic material with hook and loop fastener for easy application. The advantage of using a thermoplastic material enables the therapist to make serial adjustments as improvements in digit joint angles occur.
More recently there has been an increased debate upon the role of splinting in the treatment of Dupuytren’s Disease following a corrective procedure. Recent studies have suggested that comparable ROM results can be achieved without the routine use of splinting [22], [26], [27], [28]. Kemler [22] compared hand therapy alone (26 patients) with hand therapy and continuous splinting for four weeks removing the splint only for exercises 5 times daily (28 patients). They reported no difference between groups for ROM. However, it should be noted that continuous splinting is not the rehabilitation of choice for many hand therapists due to the inability to use the hand functionally. The debate can be further continued as there is no evidence to suggest that splints negatively affect the overall outcome [21]. Two studies [27], [28] evaluating hand therapy with night splinting compared with hand therapy alone initiated splinting in the no-splint group when extension loss of 20° PIP and 30° MCP [27] and 15° PIP and 20° MCP [28] occurred. This resulted in 10% (3/30) of patients [27] and 17% (13/69) patients [28] from the no-splint group requiring splints. Both authors advocate the use of splinting when extension loss has occurred rather than its routine use.
The option of using an extension splint may be re-evaluated and initiated according to the patient’s progress or deterioration as highlighted above. ROM is regularly measured with a goniometer to monitor for any deterioration in extension with rehabilitation adjusted accordingly. Patients with isolated MCP joint involvement may not require splinting. Conversely, recent research indicates that the PIP joint may invariably require splinting to maximise the potential gain from surgery [18], [27], [29], [30], [31], [32], [33].
When a PIP joint is involved the MCP joint may be held in a degree of flexion within the splint to minimise PIP joint tension and maximise early extension gain thus utilising the tenodesis effect [18]. The findings from the preoperative assessment and the outcomes achieved during the corrective procedure will guide rehabilitation. A contracture that is not improved during the corrective procedure will gain no further extension through rehabilitation. A contracture that has been improved actively or passively following a corrective procedure will require close monitoring so that maximum benefit from extension gained can be maintained. This is particularly so for the more complex PIP joint. Although the motion gained during the corrective procedure will guide rehabilitation, it is imperative that expectations are realistic [34].
The extension applied to the hand during exercises and splint wearing, could follow a ‘no tension’ protocol as advocated by Evans [35]. However, the results using a low load extension force for prolonged periods on contracted tissues is reported to potentially delay and reverse progression of PIP joint flexion contractures [6].
The authors preferred rehabilitation is one that follows a balance of minimal tension with promotion of digital extension and wound healing concurrently. Extension may be increased when the wound healing process permits, enabling further stretching of the joints. The authors therefore endorse the use of clinical reasoning for individual rehabilitation informed by re-assessment. Monitoring change in ROM will identify any indication for the need to splint.
Following wound healing
Oedema
Persistent oedema of the digits may compromise movement and therefore should be further managed [1], [36]. As part of usual practice, a light digital compression bandage may initially be used for intermittent periods in the day to reduce oedema. Compression bandaging as a technique has been shown to reduce oedema [37]. Additional rehabilitation modalities may involve the use of other techniques, such as contrast bathing, ultrasound and massage, to address persistent oedema. In a RCT, Hazarika [38] beneficially used an electronic intermittent pressure apparatus in order to reduce oedema and promote hand function following fasciectomy, although this treatment as presented in the study is infrequently used in practice nowadays.
Scars
It is well recognised that scar tissue formation can affect the flexibility and ROM of a joint [1], [39] and hinder function. Techniques used in hand therapy practice to reduce the effects of scarring include scar massage [20] and the application of silicone elastomer or gel [1], [33] which aim to soften the scar and increase pliability.
Movement and function
The aim of hand therapy is to increase flexion and extension in the hand to maximise function. The graded active and passive exercise programme is continued with the addition of resisted exercises to facilitate grip strength return. Accessory mobilisation of individual joints, yet to achieve the desired range, can complement previous exercises. The use of isolated DIP flexion exercises can further assist in the re-alignment of the extensor mechanism at the PIP joint. These exercises also aid full flexion for example making a fist. Lack of full flexion at the DIP of the little finger is a frequent cause of reduced grip and therefore requires attention. There is evidence to support the use of exercises after corrective intervention for Dupuytren’s Disease [16], [26] and to address persistent digital stiffness [19] with resulting benefit to grip strength [26].
The home exercise programme is extended to encourage the patient to use their hand more functionally with increased grip strength so that they can complete more demanding activities.
Splints/orthotics
Occasionally static splints are complimented by intermittent use of dynamic splints. Dynamic splints provide a passive elastic force to achieve the desired motion to gain passive, and ultimately, active ROM [31], [40]. Dynamic splints are commercially available or can be custom made. The smaller dynamic extension splints can be used to treat a single PIP joint intermittently during the day see figure 2. These enable the patients to monitor pressure on the skin and digit vascularity and for this reason are not worn during sleep. However, dynamic extension splints may compromise flexion impacting on function if not monitored correctly.

Long term
Assessments used to monitor and evaluate the hand could include physical measures of ROM and grip, PROMs and PREMs [12]. When considering PREMs it is worth noting that the patient views may be based upon the amount of extension gain alone without consideration of the benefit to function.
If used, static splints should continue to be adjusted during the rehabilitation period until a plateaux or full range is achieved. Splints are not usually continued after 3 months post operatively following its gradual withdrawal [1], [8], [9], [11], [22] or until scars are matured. Ritchie [39] reported little change in ROM after 3 months reinforcing the need to discontinue splints after this time. Patients anecdotally report continuing to maintain extension by their own means, such as sitting on their extended hand or stretching the hand following a period of flexion. Patients should be advised to monitor for signs of recurrence and instigate further intervention.
Complications and managing risk
Hand therapy rarely accounts for complications. The risks associated with compression on the skin from splints and compression bandages are minimised by putting the correct risk management strategies in place by providing adequate verbal and written instructions. The corrective intervention for Dupuytren’s Disease may lead to complications and the hand therapist plays a vital role in recognising and treating these resulting complications [10].
Occasionally, as in normal hand therapy practice, patients may experience an abnormal response to the corrective intervention leading to Complex Regional Pain Syndrome (CRPS). This requires urgent referral to the managing surgeon, and hand therapy if not already commenced.
Conclusions
Hand therapy is a complex intervention [2], [28]. Therapy management will depend upon the presentation of the patient and results of the individual hand assessment. Rehabilitation should be guided by clinical reasoning and individual need in addition to evidence based protocols.
In the past there has been a lack of evidence to support rehabilitation due to the inability of previous research to differentiate between the outcome of surgery and the results of hand therapy. More recently therapists have designed research that investigates the effectiveness of specific modalities for Dupuytren’s Disease and it is hoped that this evidence pool will continue to grow.
The decision ‘to splint’ or ‘not to splint’ remains a subject of debate. Recent therapy led research [27], [28] investigating the effectiveness of splints is challenging routine splint provision. This suggests that the routine use of night splinting is not advocated unless a loss of extension has occurred. Using their clinical reasoning the hand therapist is best placed to decide upon any splint requirements [31]. It is hoped future research into other hand therapy modalities and further research into the use of splints with the more complex PIP joint will extend this knowledge base.
References
[1] Engstrand C, Boren L, Liedberg GM. Evaluation of activity limitation and digital extension in Dupuytren's contracture three months after fasciectomy and hand therapy interventions. J Hand Ther. 2009;22(1):21-6; quiz 7. DOI: 10.1016/j.jht.2008.08.003[2] International Federation of Societies for Hand Therapy. IFSHT Hand Therapy Practice Profile 2010 [accessed Feb 2014]. Available from: http://www.ifsht.org/sites/default/files/IFSHT_Hand_Therapy_Profile_FINALJUNe%202010.pdf
[3] Pratt AL, Byrne G. The lived experience of Dupuytren's disease of the hand. J Clin Nurs. 2009;18(12):1793-802. DOI: 10.1111/j.1365-2702.2008.02692.x
[4] Markham DE, Wood MR. Ultrasound for Dupuytren's contracture. Physiotherapy. 1980;66(2):55-8.
[5] Ball C, Nanchahal J. The use of splinting as a non-surgical treatment for Dupuytren's disease: a pilot study. Br J Hand Ther. 2002;7(3):76-8.
[6] Larocerie-Salgado J, Davidson J. Nonoperative treatment of PIPJ flexion contractures associated with Dupuytren's disease. J Hand Surg Eur Vol. 2012;37(8):722-7. DOI: 10.1177/1753193411422680
[7] Christie WS, Puhl AA, Lucaciu OC. Cross-frictional therapy and stretching for the treatment of palmar adhesions due to Dupuytren's contracture: a prospective case study. Man Ther. 2012;17(5):479-82. DOI: 10.1016/j.math.2011.11.001
[8] Misra A, Jain A, Ghazanfar R, Johnston T, Nanchahal J. Predicting the outcome of surgery for the proximal interphalangeal joint in Dupuytren's disease. J Hand Surg Am. 2007;32(2):240-5. DOI: 10.1016/j.jhsa.2006.11.015
[9] Draviaraj KP, Chakrabarti I. Functional outcome after surgery for Dupuytren's contracture: a prospective study. J Hand Surg Am. 2004;29(5):804-8. DOI: 10.1016/j.jhsa.2004.05.005
[10] Prosser R, Conolly WB. Complications following surgical treatment for Dupuytren's contracture. J Hand Ther. 1996;9(4):344-8. DOI: 10.1016/S0894-1130(96)80040-8
[11] Donaldson OW, Pearson D, Reynolds R, Bhatia RK. The association between intraoperative correction of Dupuytren's disease and residual postoperative contracture. J Hand Surg Eur Vol. 2010;35(3):220-3. DOI: 10.1177/1753193409353849
[12] Ball C, Pratt AL, Nanchahal J. Optimal functional outcome measures for assessing treatment for Dupuytren's disease: a systematic review and recommendations for future practice. BMC Musculoskelet Disord. 2013;14:131. DOI: 10.1186/1471-2474-14-131
[13] Karabeg R, Jakirlic M, Arslanagic S, Dujso V, Obradovic G, Zeco A. Results of surgery treatment of Dupuytren's contracture in 115 patients. Med Arh. 2012;66(5):329-31. DOI: 10.5455/medarh.2912.66.329-331
[14] Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013;38(1):2-11. DOI: 10.1016/j.jhsa.2012.10.008
[15] Gelman SE, Schlenker R, Jacoby SM, Shin EK, Culp RW. Minimally invasive partial fasciectomy for Dupuytren contractures. Tech Hand Up Extrem Surg. 2012;16(4):184-6. DOI: 10.1097/BTH.0b013e31826246f2
[16] Herweijer H, Dijkstra PU, Nicolai JP, Van der Sluis CK. Postoperative hand therapy in Dupuytren's disease. Disabil Rehabil. 2007;29(22):1736-41. DOI: 10.1080/09638280601125106
[17] Griffin JW, Newsome LS, Stralka SW, Wright PE. Reduction of chronic posttraumatic hand edema: a comparison of high voltage pulsed current, intermittent pneumatic compression, and placebo treatments. Phys Ther. 1990;70(5):279-86.
[18] Skirven TM, Bachoura A, Jacoby SM, Culp RW, Osterman AL. The effect of a therapy protocol for increasing correction of severely contracted proximal interphalangeal joints caused by dupuytren disease and treated with collagenase injection. J Hand Surg Am. 2013;38(4):684-9. DOI: 10.1016/j.jhsa.2013.01.038
[19] Midgley R. Use of casting motion to mobilize stiffness to regain digital flexion following Dupuytren's fasciectomy. Hand Therapy. 2010;15(2):45-51. DOI: 10.1258/ht.2010.010008
[20] Sampson SP, Badalamente MA, Hurst LC, Dowd A, Sewell CS, Lehmann-Torres J, et al. The use of a passive motion machine in the postoperative rehabilitation of Dupuytren's disease. J Hand Surg Am. 1992;17(2):333-8. DOI: 10.1016/0363-5023(92)90416-M
[21] Sweet S, Blackmore S. Surgical and therapy update on the management of Dupuytren's disease. J Hand Ther. 2013. DOI: 10.1016/j.jht.2013.10.006
[22] Kemler MA, Houpt P, van der Horst CM. A pilot study assessing the effectiveness of postoperative splinting after limited fasciectomy for Dupuytren's disease. J Hand Surg Eur Vol. 2012;37(8):733-7. DOI: 10.1177/1753193412437631
[23] Bayat A, McGrouther DA. Management of Dupuytren's disease--clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88(1):3-8. DOI: 10.1308/003588406x83104
[24] Elliot D. The early history of contracture of the palmar fascia. Part 2: The revolution in Paris: Guillaume Dupuytren: Dupuytren's disease. J Hand Surg Br. 1988;13(4):371-8. DOI: 10.1016/0266-7681(88)90158-1
[25] Larson D, Jerosch-Herold C. Clinical effectiveness of post-operative splinting after surgical release of Dupuytren's contracture: a systematic review. BMC Musculoskelet Disord. 2008;9:104. DOI: 10.1186/1471-2474-9-104
[26] Glassey N. A study of the effect of night extension splintage on post-fasciectomy Dupuytren's patients. Br J Hand Ther. 2001;6(3):89-94.
[27] Collis J, Collocott S, Hing W, Kelly E. The effect of night extension orthoses following surgical release of Dupuytren contracture: a single-center, randomized, controlled trial. J Hand Surg Am. 2013;38(7):1285-94.e2. DOI: 10.1016/j.jhsa.2013.04.012
[28] Jerosch-Herold C, Shepstone L, Chojnowski AJ, Larson D, Barrett E, Vaughan SP. Night-time splinting after fasciectomy or dermo-fasciectomy for Dupuytren's contracture: a pragmatic, multi-centre, randomised controlled trial. BMC Musculoskelet Disord. 2011;12:136. DOI: 10.1186/1471-2474-12-136
[29] Van Giffen N, Degreef I, De Smet L. Dupuytren's disease: outcome of the proximal interphalangeal joint in isolated fifth ray involvement. Acta Orthop Belg. 2006;72(6):671-7.
[30] Abbiati G, Delaria G, Saporiti E, Petrolati M, Tremolada C. The treatment of chronic flexion contractures of the proximal interphalangeal joint. J Hand Surg Br. 1995;20(3):385-9. DOI: 10.1016/S0266-7681(05)80099-3
[31] Azad SM, Cherian A, Raine C, Dixon JE, Irvine BE, Erdmann MWH. Dupuytren's disease: an overview of aetiology, pathology and treatment. Br J Hand Ther. 2001;6(3):73-8.
[32] Beyermann K, Prommersberger KJ, Jacobs C, Lanz UB. Severe contracture of the proximal interphalangeal joint in Dupuytren's disease: does capsuloligamentous release improve outcome? J Hand Surg Br. 2004;29(3):240-3. DOI: 10.1016/j.jhsb.2004.02.002
[33] Rives K, Gelberman R, Smith B, Carney K. Severe contractures of the proximal interphalangeal joint in Dupuytren's disease: results of a prospective trial of operative correction and dynamic extension splinting. J Hand Surg Am. 1992;17(6):1153-9. DOI: 10.1016/S0363-5023(09)91084-X
[34] Evans RB. Therapeutic management of Dupuytren's contracture. In: Skirven TM, Osterman AL, Fedorczyk J, Amadio P, eds. Rehabilitation of the Hand and Upper Extremity Vol 1. 6th ed. Philadelphia, PA: Elsevier/Mosby; 2011. p. 1-8.
[35] Evans RB, Dell PC, Fiolkowski P. A clinical report of the effect of mechanical stress on functional results after fasciectomy for Dupuytren's contracture. J Hand Ther. 2002;15(4):331-9. DOI: 10.1016/S0894-1130(02)80004-7
[36] Glassey N, Phillips M. Comparison of the effect of two application methods of self-adherent wrap (Coban™) on range of motion in the hands of healthy subjects. Hand Therapy. 2011;16(2):42-4. DOI: 10.1258/ht.2011.011004
[37] Lowell M, Pirc P, Ward RS, Lundy C, Wilhelm DA, Reddy R, Held B, Bernard J. Effect of 3M Coban Self-Adherent Wraps on edema and function of the burned hand: a case study. J Burn Care Rehabil. 2003 Jul-Aug;24(4):253-8; discussion 252. DOI: 10.1097/01.bcr.0000075846.92114.ad
[38] Hazarika EZ, Knight MT, Frazer-Moodie A. The effect of intermittent pneumatic compression on the hand after fasciectomy. The Hand. 1979;11(3):309-14. DOI: 10.1016/S0072-968X(79)80056-X
[39] Ritchie JF, Venu KM, Pillai K, Yanni DH. Proximal interphalangeal joint release in Dupuytren's disease of the little finger. J Hand Surg Br. 2004;29(1):15-7. DOI: 10.1016/j.jhsb.2003.08.005
[40] Ebskov LB, Boeckstyns ME, Sorensen AI, Soe-Nielsen N. Results after surgery for severe Dupuytren's contracture: does a dynamic extension splint influence outcome? Scand J Plast Reconstr Surg Hand Surg. 2000;34(2):155-60. DOI: 10.1080/02844310050160024



