Urinary microbiota analyzed by extended microbiology in subjects with and without urological disorders
Michael Kogan 2
1 Department of Microbiology, Rostov State Medical University, Rostov on Don, Russland
2 Department of Urology, Rostov State Medical University, Rostov-on-Don, Russland
Abstract
A healthy person’s urine is not a sterile environment, and its microbiota contain a wide range of Gram-positive and Gram-negative aerobic and anaerobic bacteria, with Gram-positive aerobic microorganisms predominating. There are significant differences between the urinary microbiota of healthy men and women. The composition of healthy women’s urinary microbiota depends on age and sexual activity. Non-clostridial anaerobic bacteria (NAB) predominate Enterobacteriaceae in case of acute obstructive pyelonephritis with bacteriuria of 104 CFU/ml and higher. Experiments have shown that obstructive pyelonephritis can be caused by various species of NAB (Peptococcus spp., Bacteroides spp., Eubacterium spp.). In every third case of recurrent symptomatic uncomplicated lower urinary tract infection (UTI) Enterobacteriaceae with low level bacteriuria of ≤103 CFU/ml are found and every third case of acute cystitis is not associated with typical uropathogens. Low levels of bacteriuria (102–103 CFU/ml) correlate with symptoms of upper and lower UTI and thus cannot be considered contamination. Asymptomatic bacteriuria is normal in healthy individuals.
Summary of recommendations
- A sample of urine for bacteriological examination should be collected minimizing contamination of the material, and culture of urine should be conducted on an expanded set of culture media. To identify the facultative anaerobic bacteria it is advisable to use MacConkey Agar, HiCrome Candida Differential Agar, HiCrome Enterococci Agar, HiCrome Aureus Agar Base, Blood Agar prepared according to Mueller, Hinton agar added with sheep erythrocytes, and in order to identify non-clostridial anaerobic bacteria to use Anaerobic Agar, Broth Shaedler, Shaedler Agar, Bacteroides Bile Esculinum Agar, MRS Agar.
- Culture medium for the cultivation of anaerobic and microaerophilic bacteria should be used for bacteriological urine culture in acute and chronic infections of the upper and lower urinary tracts.
- In acute obstructive pyelonephritis, a level of bacteriuria for Enterobacteriaceae equal to 103–104 CFU/ml should be considered clinically significant.
- In the experimental obstruction of the ureter, several taxons of non-clostridial anaerobic bacteria (Peptococcus spp., Bacteroides spp., Eubacterium spp.) 105 CFU/ml lead to the development of acute suppurative lesions of the kidney. In this regard, non-clostridial anaerobic bacteria should be considered as an etiological factor of acute obstructive pyelonephritis.
- Difficult to cultivate microorganisms at a level of bacteriuria <104 CFU/ml should be considered etiologic for acute obstructive pyelonephritis if typical uropathogens are absent.
- If non-clostridial anaerobic bacteria are isolated at a level of ≤103 CFU/ml and typical uropathogens are absent in the urine of patients with uncomplicated infection of the lower urinary tract, their causative role cannot be ruled out.
- Gram-positive microorganisms and non-clostridial anaerobic microorganisms in voided or catheterized urine samples at levels of 102–103 CFU/ml, should not be considered contamination.
- Asymptomatic bacteriuria is normal in healthy individuals.
1 Introduction
Standard microbiological protocols for investigation of urine include microscopic examination with Gram stain and urine culture using media such as Blood and MacConkey Agar incubated at aerobic conditions (35°С, 24 hours) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. Such investigations aim to identify microorganisms in the urine known to cause urinary tract infection (UTI). These are mainly species of Enterobacteriaceae (Escherichia coli, Klebsiella spp., Proteus spp., et al.), Pseudomonas spp. and some selective Gram-positive species (Staphylococcus aureus, S. saprophyticus, Enterococcus spp., et al.). These standard protocols, however, narrow down the possible spectrum of uropathogens. Based on the ensuing results new antibacterial agents are developed and manufactured. Common knowledge about uropathogens remained unchanged until recently, when some studies showed, that some microorganisms, so far unknown as uropathogens, may also cause UTI [12], [13], [14], [15], [16], [17], [18].
As early as 1939 Schulte et al. [19] isolated Bacteroides spp. and anaerobic streptococci in patients with unilateral pyelonephritis. Later in 1976 Japanese authors [20] created an experimental model of acute pyelonephritis caused by B. fragilis, proving morphologically the etiologic role of this organism in inflammatory kidney lesions. Sapico et al. [21] isolated some taxons of non-clostridial anaerobic bacteria (NAB), such as Bifidobacterium spp. and Veillonella spp., from the bladder urine of patients with permanent indwelling urethral catheter. Brook [22] isolated Bacteroides spp., Peptococcus spp., and Bifidobacterium spp. from the urine of children with acute pyelonephritis and cystitis and as early as 1980 indicated the necessity of using anaerobic bacteriological studies. Apostolopoulo et al. [23] also noticed the relationship between NAB and development of kidney infections, because NAB were identified in 24.4% of kidney pathology samples after nephrectomy and 22.2% of the kidney bioptates and bladder urine with the predominance of Bacteroides spp. DuPrey et al. [24] described a case of acute pyelonephritis caused by Lactobacillus delbrueckii with a level of bacteriuria of >1010 CFU/mL. The patient had a history of type II diabetes mellitus and hypothyreoidism.
In the 1990s our investigators’ group extended the spectrum of culture media, including anaerobic culturing techniques in order to increase the isolation of a wider spectrum of aerobic and anaerobic bacteria. Those media were previously used to study vaginal and bowel microbiota [1], [2], [3], [10]. We could show [25], [26], [27], [28], [29], [30] that urine of healthy men, women and children and urine of patients with UTI contains a wide spectrum of aerobic and anaerobic bacteria and also virus-bacteria associations.
Finally the realization of the Project to Study the Human Microbiome (2008–2012) became a serious breakthrough in proving that urine of healthy individuals and of patients with UTI is colonized by many different microorganisms with different levels of bacteriuria [4], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]. New impulses for a modern view on urinary microbiota were generated by Brubaker [7]. He stated that “the standard urine culture protocol was not designed to detect bacteria that require special nutrients, grow slowly, cannot tolerate oxygen, or are present in small numbers (<103 CFU/mL). Some of these organisms may be involved in urinary disorders”. Aagaard [41] critised standards even sharper: “Even if a single microbe is the etiologic agent of infection, the pathogenesis and pathophysiology of infection can be viewed within the context of the microbiome and human biology”, and further “Connections among microbiomes in different body sites may help us to understand patterns of human infections”.
Now, we have entered a new era to investigate the urinary microbiota of healthy subjects and patients suffering from UTI. This will lead us to a new understanding of etiology, pathophysiology and management of UTI in human.
2 Methods
A systematic literature search was performed for the last 10 years in MEDLINE, PubMed, ClinicalKey, Cochrane for the following key words: microbiota urine, urine culture, culture urine, culture medium for the examination of the urine, urinary tract infection and the following limitations: age, children, pregnant women, clinical studies, English, abstract available, only peer reviewed.
A total of 1,189 publications was found and screened by title and abstract. After exclusion of duplicates a total of 50 publications was included into the review.
3 Results
3.1 Urinary microbiota of healthy women
The paradigm on the sterility of a healthy person’s urine was based for more than half a century only on the bacteriological methods to investigate urine samples. We have changed this approach by using both, the conventional culture media (blood and MacConkey agar) and also a wide range of additional culture media to detect also facultative anaerobic bacteria (FAB) and non-clostridial anaerobic bacteria (NAB). These media are commonly used for identification of microorganisms derived from colon and vagina. For the FAB cultivation we used MacConkey Agar, HiCrome Candida Differential Agar, HiCrome Enterococci Agar, HiCrome Aureus Agar Base, Blood Agar, prepared according to Mueller Hinton agar with addition of sheep red blood cells. For the NAB cultivation we used Anaerobic Agar, Shaedler Broth, Shaedler Agar, Bacteroides Bile Esculinum Agar, MRS Agar. Nutritional media were purchasedfrom HiMedia (India). The culture media were incubated with the test material under aerobic (24–48 hours) and anaerobic (48–72 hours) conditions (10% СО2, 10% Н2, 80% N2).
To study the microbiota of normal urine, 66 healthy women were divided into three groups:
- group I – 22 not sexually active women (18–25 years)
- group II – 24 sexually active women (18–25 years)
- group III – 20 sexually active women in the postmenopause (52–65 years)
All women were healthy according to medical history, general physical examination, and vital signs. Especially they had no urological and gynecological disorders including ultrasound examination of the internal genitalia. They had no infectious diseases during the last year, and were without medication for the last 2 months. In group III the menopause occurred before 5 years or earlier, without any pelvic organ prolapse, with the uterus still present and with at least two deliveries without abortion in the history. Complete blood count and urinalysis were normal in all women.
From each subject midstream samples of morning urine were collected for bacteriological examination, which was repeated three times with an interval of 3 days. The urine samples were collected in a way to minimize the risk of contamination with microorganisms from the skin, periurethral area and vagina. A total of 198 urine samples was studied.
After a threefold bacteriological examination of urine samples from healthy women of the groups I, II, III, no sterile sample was found in any case. Microorganisms were detected in all urine samples with different species at different proportions between FAB and NAB (Table 1).
| Microorganisms | group I | group II | group III | |||
|---|---|---|---|---|---|---|
| % | CFU/mL |
% | CFU/mL | % | CFU/mL | |
| Facultative anaerobic bacteria (FAB) | ||||||
| CNS | 90.9 | 102 | 83.8 | 103 | 60.0* | 102 |
| Corynebacterium spp. | 90.9 | 102 | 75.0* | 102 | 50.0* | 102 |
| Enterococcus spp. | 36.4 | 102 | 12.5* | 102 | 70.0* | 102 |
| Micrococcus spp. | 18.2 | 102 | 12.5 | 102 | 20.0* | 102 |
| Klebsiella spp. | 18.2 | 102 | 0 | 0 | 0 | 0 |
| S. aureus | 18.2 | 102 | 16.7 | 102 | 0 | 0 |
| Streptococcus spp. | 9.1 | 102 | 8.3 | 102 | 0 | 0 |
| Candida spp. | 9.1 | 102 | 33.3* | 102 | 0 | 0 |
| Bacillus spp. | 0 | 102 | 20.8 | 102 | 0 | 0 |
|
E. coli |
0 | 102 | 16.7 | 102 | 20.0 | 104 |
| Non-clostridial anaerobic bacteria (NAB) | ||||||
| Lactobacillus spp. | 81.8 | 104 | 83.3 | 104 | 10.0* | 102 |
| Peptococcus spp. | 72.7 | 103 | 75.0 | 103 | 45.0* | 102 |
| Propionibacterium spp. | 72.7 | 103 | 58.3* | 104 | 50.0 | 102 |
| Eubacterium spp. | 54.5 | 104 | 41.7* | 105 | 50.0 | 102 |
| Peptostreptococcus spp. | 45.5 | 103 | 41.7 | 103 | 60.0* | 102 |
| Bacteroides spp. | 18.2 | 103 | 25.0 | 104 | 0 | |
| Veillonella spp. | 0 | 0 | 16.7 | 103 | 40.0* | 102 |
| Prevotella spp. | 0 | 0 | 12.5 | 102 | 15.0 | 102 |
| Actinomyces spp. | 0 | 0 | 8.3 | 102 | 0 | |
| Megasphaera | 0 | 0 | 0 | 0 | 55.0 | 102 |
| Mobiluncus spp. | 0 | 0 | 0 | 0 | 20.0 | 103 |
| Fusobacterium spp. | 0 | 0 | 0 | 0 | 20.0 | 102 |
|
Note: |
||||||
For urine of healthy women of all age groups, the dominance of coagulase-negative staphylococci (CNS), Corynebacterium spp., Peptococcus spp., Propionibacterium spp., Eubacterium spp., and Peptostreptococcus spp. was typical. In the urine of postmenopausal women, Enterococcus spp. are more frequent (p<0.05). The dominance of Lactobacillus spp. in urine of young women (group I and II) is opposed by their rare (10%) detection in urine of postmenopausal women. E. coli, Veillonella spp., Prevotella spp. appeared more frequently in the urine of sexually active women and postmenopausal women than in the urine of sexually not active young women. Some FAB species were not found in the urine of healthy postmenopausal women. The lowest detectable degree of FAB bacteriuria (102 CFU/ml) was found for almost all microorganisms, except for E. coli in postmenopausal women (104 CFU/ml). On the contrary, the level of bacteriuria for most of NAB for young women was between 103 and 105 CFU/ml and for postmenopausal women this level was reduced to the lowest detectable level (102 CFU/ml).
In an unpublished study we conducted a study comparing three midstream urine samples obtained at 8.00, 12.00, 16.00 hours in a single day from 20 healthy, sexually active women aged 18–20 years. In general insignificant daily fluctuations of frequencies and levels of bacteriuria were found (Figure 1) with the exception of certain genera, as confirmed by cluster analysis.
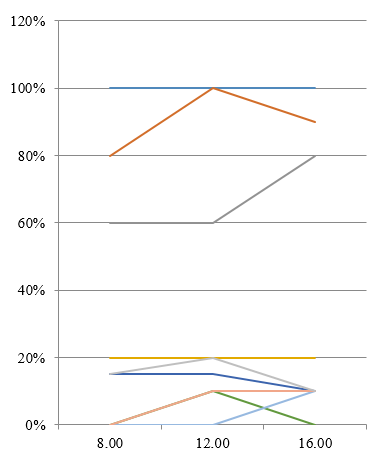
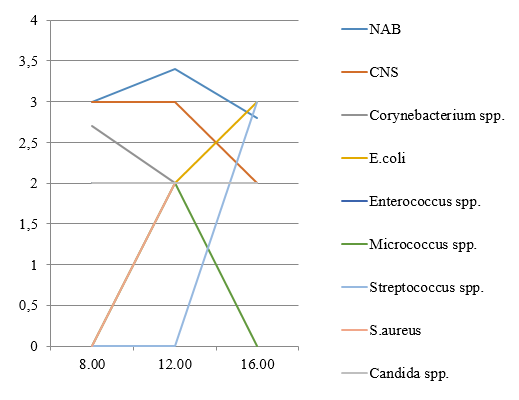
Figure 1: Frequency of detection (%) and average number (lg CFU/ml) of microorganisms detected from healthy women’s urine.
In conclusion, the urine of healthy women of all age groups is not sterile and is dominated by Gram-positive and not Gram-negative species of FAB and NAB.
3.2 Urinary microbiota of healthy men
Midstream morning urine samples were collected three times with an interval of 3 days from 20 sexually active, healthy (criteria see above) men aged 20–25 years and cultivated on the expanded set of culture media as mentioned above (Table 2).
| Microorganisms | Frequency of detection (%) | The average level of bacteriuria (CFU/ml) |
| Facultative anaerobic bacteria (FAB) | ||
| CNS | 89.3 | 102 |
| Corynebacterium spp. | 78.6 | 102 |
| Enterococcus spp. | 50.0 | 102 |
| E. coli | 10.7 | 102 |
| S. aureus | 10.7 | 102 |
| Non-clostridial anaerobic bacteria (NAB) | ||
| Eubacterium spp. | 78.6 | 103 |
| Peptostreptococcus spp. | 50.5 | 102 |
| Bacteroides spp. | 21.4 | 103 |
| Peptococcus spp. | 21.4 | 102 |
| Megasphaera | 21.4 | 102 |
| Propionibacterium spp. | 10.7 | 102 |
| Veillonella spp. | 10.7 | 102 |
| Mobiluncus spp. | 10.7 | 103 |
| Fusobacterium spp. | 10.7 | 102 |
In the urine of healthy men there is a prevalence of CNS, Corynebacterium spp., and Enterococcus spp. among the FAB, and Eubacterium spp., and Peptostreptococcus spp. among the NAB. The NAB patterns ranged twice as wide as compared with the FAB patterns. The levels of bacteriuria for FAB and NAB were at the lower level of detection.
3.3 Comparison between urinary microbiota of healthy women and men
The comparison was conducted for the young sexually active groups. The urinary microbiota of men and women were similar in the dominant frequency of CNS and Corynebacterium spp. However, the following differences were found: The FAB range found in men was twice as narrow as that found in women. The spectra of NAB in men and women differed: in men Lactobacillus spp., Actinomyces spp. were absent, but Megasphaera spp., Mobiluncus spp., and Fusobacterium spp. could be detected in contrast to women.
The cluster analysis of the microbiological results revealed typical “female” and “male” urinary microbiota spectra (Figure 2). In mixed clusters men were more similar to men than men to women. The majority of female urinary microbiota (66.6 per cent) were found in mixed clusters, which means higher differences in the urinary microbiota of women than of men.
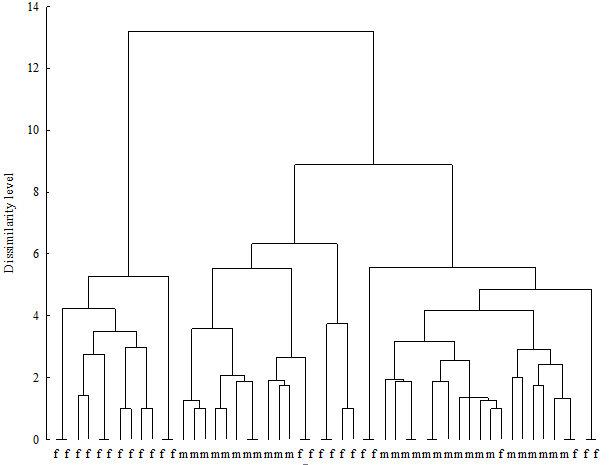
Figure 2: Hierarchical clustering of urinary microbiota in healthy men and women
Note: m - male; f - female
Our bacteriological results of urinary microbiota in healthy individuals, using an expanded set of culture media, correspond with other studies. Siddiqui et al. [42] found by high throughput sequencing of 16S RNA amplicons 45 different species of microorganisms in the female urine microbiota with predominance of Lactobacillus spp., Prevotella spp., and Gardnerella spp.
Fouts et al. [43] revealed the dominant role of Corynebacterium spp. in the urinary microbiome of healthy men. Lactobacillus spp., Corynebacterium spp., Gardnerella spp., Prevotella spp., and Enterococcus spp. showed differences between both genders. These results suggest that the state of healthy urine is, in fact, one of “asymptomatic bacteriuria”. In our studies presented above urine of healthy sexually active men revealed also a prevalence of FAB with CNS (89.3%) and Corynebacterium spp. (78.6%).
In a study by Wolfe et al. [33] the bacterial spectra of urine collected during normal urination, through a transurethral catheter (TUC) and taken by suprapubic aspiration (SPA) were compared. The presence of bacteria in these samples was assessed by bacterial culture, light microscopy, and 16S rRNA gene sequencing. Fourteen genera out of 321 total (5%) in samples obtained by TUC and SPA were present in different relative abundances. Of those 14 genera, only Lactobacillus represented more than 1% of the total sequences present in either the TUC (26.96%) or SPA (6.94%) samples. The most abundant genera found for TUC samples were similar to those found for SPA urine samples.
From their results the authors concluded that standard clinical microbiological procedures favor detection of fast-growing bacteria only in the presence of oxygen. They consistently underestimate slow-growing bacteria and can detect neither anaerobic bacteria nor those whose preferred growth conditions remain unknown. As an example the authors discussed a clinical observation. that urine, obtained from a participant and determined by standard cultivation procedures to be positive for E. coli, was rich in DNA associated with the fastidious genera Aerococcus (40% and 50% in SPA and TUC, respectively) and Actinobaculum (25% and 17%) yet poor in DNA identified with E. coli (3% and 1%). The disparity between the culture and molecular assays raised the question: are this woman’s clinical symptoms due to the relatively small number of the “typical” uropathogenic E. coli, the numerically superior fastidious bacteria, or a combination of both?
In this context the authors stated further, that “the likelihood that diverse bacteria can exist within the female urinary tract has important implications for urinary tract disorder researchers. These findings should stimulate work to advance our understanding of the roles played by these bacterial communities in the development of UTI and other urinary tract disorders that remain poorly understood. Such studies could make it possible to identify at-risk UTI populations and allow hypothesis-based research of improved targeted treatments and/or prevention efforts”.
These studies, including ours, may provide a new prospect on the concept of “normal (asymptomatic) bacteriuria”, and on additional infectious agents causing inflammatory diseases of the urinary tract.
3.4 Acute complicated (obstructive) pyelonephritis
The majority (>90%) of patients with acute obstructive pyelonephritis present with bacteriuria of ≥105 CFU/ml of Enterobacteriaceae or some Gram-positive species (S. aureus, S. saprophyticus, Enterococcus spp.) as the main etiological pathogens. Since according to our studies urine of healthy individuals does not only contain species of Enterobacteriaceae, but also a wide spectrum of CNS, Corynebacterium spp., and NAB, the involvement of these microorganisms in the development and clinical course of acute obstructive pyelonephritis (AOP) may be of interest. Consequently we studied the urinary microbiota of the renal pelvis immediately after release of the ureter obstruction.
Our research [44] included 72 patients (18–74 years) with AOP, caused by ureteral stone with obstruction lasting 1–14 days. For culture urine from the bladder was obtained by catheterization and from the renal pelvis by ureteral catheter or percutaneous nephrostomy immediately after release of the ureteral obstruction. Bacteriological examination of urine was performed using an expanded set of culture media as mentioned above.
Study results of the microbiota found in the bladder and renal pelvis urine are presented in Table 3.
| Microorganisms | Bladder urine (n – 72) |
Renal pelvis urine (n – 68) |
||||
| % | CFU/mL (range) | CFU/mL (mean) | % | CFU/mL (range) | CFU/mL (mean) | |
| Enterobacteriaceae | 80.5 | 102–108 | 104.7 | 80.8 | 102–107 | 104.8 |
| E. coli | 52.7 | 102–108 | 104.9 | 52.9 | 102–107 | 104.7 |
| Klebsiella spp. | 19.4 | 102–107 | 104.4 | 19.1 | 102–106 | 104.1 |
| Proteus spp. | 6.9 | 104–105 | 104.6 | 7.3 | 103–105 | 104.4 |
| Providencia rettgeri | 1.3 | 105 | 105.0 | 1.4 | 103 | 103.0 |
| Nonfermentative Gram-negative bacteria | ||||||
| Burkholderia cepacia | 1.3 | 106 | 106.0 | 1.4 | 105 | 105.0 |
| Gram-positive flora | 63.8 | 102–106 | 102.8 | 52.9 | 102–106 | 102.1 |
| CNS | 44.4 | 102–106 | 103.2 | 27.9* | 102–106 | 103.4 |
| Corynebacterium spp. | 30.5 | 102–105 | 102.7 | 16.1* | 102–104 | 102.6 |
| Enterococcus spp. | 30.5 | 102–105 | 103.1 | 19.1* | 102–103 | 102.4 |
| S. aureus | 5.5 | 102 | 102.0 | 7.3 | 102 | 102.0 |
| Micrococcus spp. | 1.3 | 102 | 102.0 | - | - | - |
| Bacillus sp. | 1.3 | 102 | 102.0 | - | - | - |
| NAB | 94.4 | 102–105 | 102.4 | 89.7 | 102–106 | 103.0 |
| Eubacterium spp. | 50.5 | 102–105 | 103.1 | 39.7* | 102–105 | 103.2 |
| Peptococcus spp. | 36.1 | 102–105 | 102.4 | 27.9* | 102–106 | 103.5 |
| Peptostreptococcus spp. | 30.5 | 102–105 | 102.8 | 22.0* | 102–105 | 103.9 |
| Propionibacterium spp. | 29.1 | 102–105 | 102.2 | 17.6* | 102–104 | 103.2 |
| Bacteroides spp. | 13.8 | 102–105 | 102.3 | 13.2 | 102–105 | 103.3 |
| Fusobacterium spp. | - | - | - | 5.8 | 102 | 102.0 |
| Veillonella spp. | - | - | - | 4.4 | 102 | 102.0 |
| Megasphaera spp. | 1.3 | 102 | 102.0 | - | - | - |
| Lactobacillus spp. | 16.7 | 102–103 | 102.5 | 16.7 | 102–103 | 102.5 |
| Fungal flora | ||||||
| Candida tropicalis | 1.3 | 102 | 102.0 | 1.4 | 102 | 102.0 |
|
Note: |
||||||
The study showed a high frequency (81.8%) of Gram-negative bacteria in the bladder urine at AOP, which are common uropathogens. To a lesser extent (63.8%) a wide range of Gram-positive bacteria was identified in the urine. The highest frequency (94.4%) was demonstrated for NAB. It is remarkable that the level of bacteriuria for Enterobacteriaceae had a wide range (102–108 CFU/mL) and only in 30.5% of cases a level of ≥105 CFU/ml, and in 49.2% a level of ≥104 CFU/ml was found. At the same time a high level of bacteriuria (≥104 CFU/ml) was found in 8.8% for Gram-positive bacteria (CNS, Corynebacterium spp., and Enterococcus spp.) and 31.3% for NAB.
The microbiota of the bladder and renal pelvis urine were comparable concerning spectrum, frequency and level of bacteriuria for Enterobacteriaceae. The Gram-positive microflora and NAB, however, showed a significantly reduced frequency in the renal pelvis urine, but concerning the five main NAB species the average level of bacteriuria in the renal pelvis was higher than the level of the bladder bacteriuria. The bacteriuria levels of Gram-positive bacteria were similar for bladder and renal pelvis urine.
Thus, the study confirms the validity of the bacteriological evaluation of bladder urine for the detection of pathogens, related to the development of acute pyelonephritis after ureteral obstruction.
In all of the 72 cases the urinary microbiota included three or more taxons of microorganisms. In 94.4% of the cases an aerobic-anaerobic composition was found, but in only 5.6% a purely aerobic one. In 29.2% of the cases, the level of bacteriuria was ≥104 CFU/ml for at least 2 taxons. Considering the wide range of microorganisms found in the urine of the renal pelvis, the question arises, which of the taxons finally initiated or potentiated the pyelonephritis?
3.5 Experimental modeling of acute obstructive pyelonephritis
Using the AOP animal model published by Giamarellos-Bourboulis et al. [45] 60 male rabbits of the New Zealand breed were randomly divided into six groups of 10 animals each. Bacterial suspension of NAB (volume 1 ml containing 105 CFU/ml) obtained from AOP patients were injected into the renal pelvis of the animals:
- Group 1 – E. coli
- Group 2 – Peptococcus spp.
- Group 3 – Eubacterium spp.
- Group 4 – Propionibacterium spp.
- Group 5 – Bacteroides spp.
- Group 6 – E. coli + Peptococcus spp.
The animals were killed on day 1, 3, 7, 14 and 21 of the experiment. Pathomorphologic studies of the kidney samples were performed using tissue preparations stained with hematoxylin-eosin.
Experimental AOP caused by E. coli showed typically morphological alterations [46], [47] and served as control.
All animals in groups 2–6 developed morphological features of acute pyelonephritis. As a result of ureteral obstruction in all animals an acute kidney inflammatory reaction took place already on the first day of the experiment. In group 2 on the first day pyelitis and peripyelitis were observed. On days 2–3 a septic phlebitis and arteritis developed, and on days 3–7 foci of papillomatosis and infarctions of the cortex and medulla were observed.
In group 3 formation of microabscesses was detected on the first day, and on the third day heart attacks, papillary necrosis and contralateral kidney damage occured. In group 4, serofibrinous pelvis inflammation was registered from the 1st day, from 1st–3rd day pyelitis and peri-pyelitis, and from the 3rd–7th day minimum inflammatory reactions of the renal medulla were seen. In group 5, acute inflammation of the renal interstitial tissue (medulla) developed on the 1st day, and on the 3rd–7th day microabscesses of the renal parenchyma were seen. In group 6 from the 1st day onwards, pyelitis, peri-pyelitis, a serious suppurative inflammation of the medulla, and septic thrombophlebitis were noted, on the 3rd–7th days hemorrhagic necroses of the renal parenchyma developed, and all animals died up to the 14th day.
Thus, in experimental animals with ureteral obstruction, NAB inoculated into the renal pelvis reproduced morphological features of acute purulent inflammation of the renal pelvis and renal parenchyma. Strains of Propionibacterium spp. basically provoked pyelitis and peri-pyelitis with a minimal involvement of kidney parenchyma, but Peptococcus spp. provoked the development of inflammatory reactions similar to E.coli. Monovariant Eubacterium spp. and Bacteroides spp. cause more severe alterations of kidney destruction compared to alterations caused by E. coli. Mixed infection E. coli + Peptococcus spp. was responsible for the most severe kidney destruction in form of abscesses, heart attacks, hemorrhagic necrosis and finally animals’ death.
The severity of (ascending) renal damage in the animal experiments could be ranked as shown in Figure 3:
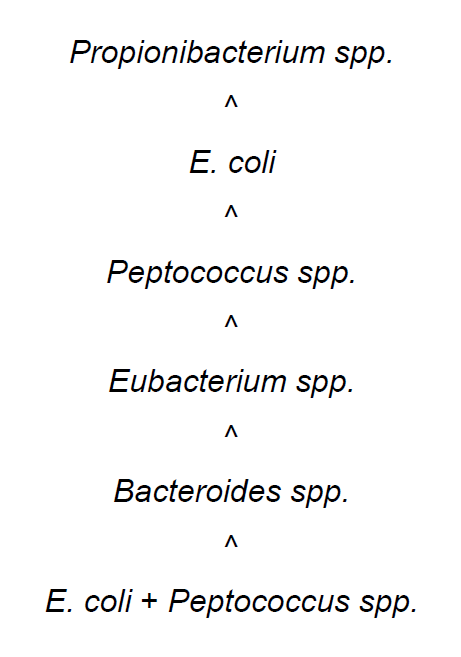
renal damage in the animal experiment
3.6 Urine microbiota in women with recurrent uncomplicated lower urinary tract infections (ULUTI)
In 25–30% of cases acute cystitis is caused by Enterobacteriaceae and by some Gram-positive species with a level of bacteriuria <105 CFU/ml [48], [49], [50]. According to the recommendations of the EAU bacteriuria ≥104 CFU/ml is considered diagnostically significant in symptomatic recurrent infections of the lower urinary tract [51]. The same uropathogenic bacteria are considered to cause pyelonephritis.
We performed bacteriological examination of the morning midstream urine from 144 women (20–50 years) with an acute cystitis of recurrent ULUTI (Table 4). Duration of recurrent ULUTI: <1 year (14.3%), 2–4 years (35.4%) and >5 years (50.3 %). All patients had been previously treated repeatedly with antibiotics.
| Microorganisms | The frequency of detection (%) | Range (CFU/ml) | Meanlevel of bacteriuria (CFU/ml) |
| Enterobacteriaceae | |||
| E. coli | 35.5 | 102–106 | 104.2 |
| Klebsiella spp. | 9.7 | 102–106 | 104.0 |
| Enterobacter spp. | 9.1 | 102–105 | 103.4 |
| Citrobacter spp. | 6.9 | 102–107 | 103.8 |
| Proteus spp. | 4.8 | 102–107 | 105.7 |
| Providencia rettgeri | 2.1 | 106 | 106.0 |
| Nonfermentative gram-negative bacteria | |||
| Pseudomonas spp. | 4.2 | 106 | 106.0 |
| Gram-positive flora | |||
| CNS | 65.2 | 102–104 | 102.7 |
| Corynebacterium spp. | 61.1 | 102–105 | 102.5 |
| Enterococcus spp. | 28.5 | 102–103 | 102.4 |
| Streptococcus spp. | 12.5 | 102 | 102.0 |
| S. aureus | 10.4 | 102–105 | 103.3 |
| Candida spp. | 26.4 | 102–105 | 103.0 |
| NAB | |||
| Propionibacterium spp. | 50.7 | 102–104 | 102.6 |
| Peptococcus spp. | 48.6 | 102–106 | 103.2 |
| Eubacterium spp. | 42.4 | 102–105 | 103.1 |
| Lactobacillus spp. | 40.9 | 102–105 | 103.4 |
| Peptostreptococcus spp. | 29.2 | 102–105 | 103.2 |
| Bacteroides spp. | 11.1 | 102–104 | 102.7 |
| Actinomyces spp. | 7.6 | 102–104 | 102.5 |
| Fusobacterium spp. | 6.2 | 102–103 | 102.3 |
| Prevotella spp. | 5.5 | 103 | 103.0 |
The proportion of Enterobacteriaceae in the microbiota was 68.1%, while E. coli was cultured only in 35.5% of the cases. The isolation frequency of Klebsiella spp. and Enterobacter spp. from urine was higher in this than in other studies. Overall, the level of bacteriuria ≤103CFU/ml with Enterobacteriaceae was detected in 36.7% of cases, and that ≥104 CFU/ml in 63.3%. The spectrum of Gram-positive bacteria and NAB were identical with the urine microbiota of healthy women. However, their frequency was lower and the level of bacteriuria significantly higher than in healthy subjects.
Thus, the qualitative composition of the urine microbiota in female patients with acute cystitis was practically identical to that in healthy women with increasing frequency of detection and levels of bacteriuria with Enterobacteriaceae. However, it is not known whether such a low number (≤103 CFU/mL) of Enterobacteriaceae can cause acute cystitis, or whether the infection is caused by organisms of all other taxa in the presence of Enterobacteriaceae. After all in 27.7% of cases with ULUTI such uropathogens (Enterobacteriaceae, Pseudomonas spp., Enterococcus spp., S. aureus, S. saprophyticus) were not found in the urine of these patients. It seems likely that on standard culture media hardly cultivated and/or non-cultivated microorganisms may have been involved in the development of such an acute cystitis episode. With an expanded set of culture media in all cases of ULUTI aerobic-anaerobic bacterial compositions could be identified assuming that such "unproven" bacteria were the pathogens of the recurrent cystitis.
4 Further Research
Further studies are needed to elucidate the role of hardly cultivated microorganisms found in the urine of healthy individuals, in the development of symptomatic infections of upper and lower urinary tract. The levels of bacteriuria of dominant bacterial taxons should be determined and their ability to cause symptomatic infections of the upper and lower urinary tract. It is advisable to improve the standard of the classic bacteriological examination of the urine.
5 Conclusion
Polymicrobial bacteriuria should be considered normal condition of healthy individuals. The concepts of significant bacteriuria (≥105 CFU/ml) as proposed by Kass E. [52], pathogenic bacteriuria, “asymptomatic bacteriuria”, and contamination need to be reconsidered. A number of NAB species are symbiotes in the urine of healthy individuals, but can also cause acute septic renal infection, similarly to E. coli. The proof of two or more bacterial species in the urine of patients with OAP at a concentration of ≥104–5 CFU/ml allows to consider them as causative in the development of a renal inflammatory process.
6 Acknowledgement
We thank the staff of the departments of Urology and Microbiology for their help in collecting material, laboratory of bioinformatics, medical statistics and computer modeling for assistance in statistical data processing, as well as the patients for agreeing to participate in research.
References
[1] Murray PR, Rosental KS, Pfaller MA, Medical Microbiology. 7th ed. Philadelphia: Elsevier; 2013.[2] Engleberg NC, DiRita V, Dermody TS. Schaechter's Mechanisms of microbial disease. 5th ed. Philadelphia: Wolters Kluwer Lipincott Williams and Wilkins; 2013.
[3] Greenwood D, Barer M, Slack R, Irving W. Medical Microbiology - A Guide to Microbial Infections: Pathogenesis, immunity, laboratory investigation and control. Edinburgh: Churchill Livingstone/Elsevier; 2012.
[4] Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014 Mar;52(3):871-6. DOI: 10.1128/JCM.02876-13
[5] Maskell RM. The natural history of urinary tract infection in women. Med Hypotheses. 2010 May;74(5):802-6. DOI: 10.1016/j.mehy.2009.12.011
[6] Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi JR, Drake MJ. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41. DOI: 10.3389/fcimb.2013.00041
[7] Brubaker L, Wolfe AJ. The new world of the urinary microbiota in women. Am J Obstet Gynecol. 2015 Nov;213(5):644-9. DOI: 10.1016/j.ajog.2015.05.032
[8] Cary EN, Dirita V, Dermody TS. Schaechters Mechanisms of microbial disease. 5th ed. Philadelphia: Wolters Kluwer Lipincott Williams and Wilkins; 2013. p. 360.
[9] Pearce MM, Zilliox MJ, Rosenfeld AB, Thomas-White KJ, Richter HE, Nager CW, Visco AG, Nygaard IE, Barber MD, Schaffer J, Moalli P, Sung VW, Smith AL, Rogers R, Nolen TL, Wallace D, Meikle SF, Gai X, Wolfe AJ, Brubaker L; Pelvic Floor Disorders Network. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol. 2015 Sep;213(3):347.e1-11. DOI: 10.1016/j.ajog.2015.07.009
[10] Mandell GL. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. London: Elsevier; 2015.
[11] Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004 Apr;38(8):1150-8. DOI: 10.1086/383029
[12] Bank S, Hansen TM, Søby KM, Lund L, Prag J. Actinobaculum schaalii in urological patients, screened with real-time polymerase chain reaction. Scand J Urol Nephrol. 2011 Dec;45(6):406-10. DOI: 10.3109/00365599.2011.599333
[13] Bank S, Jensen A, Hansen TM, Søby KM, Prag J. Actinobaculum schaalii, a common uropathogen in elderly patients, Denmark. Emerging Infect Dis. 2010 Jan;16(1):76-80. DOI: 10.3201/eid1601.090761
[14] Cattoir V. Actinobaculum schaalii: review of an emerging uropathogen. J Infect. 2012 Mar;64(3):260-7. DOI: 10.1016/j.jinf.2011.12.009
[15] Reinhard M, Prag J, Kemp M, Andresen K, Klemmensen B, Højlyng N, Sørensen SH, Christensen JJ. Ten cases of Actinobaculum schaalii infection: clinical relevance, bacterial identification, and antibiotic susceptibility. J Clin Microbiol. 2005 Oct;43(10):5305-8. DOI: 10.1128/JCM.43.10.5305-5308.2005
[16] Darbro BW, Petroelje BK, Doern GV. Lactobacillus delbrueckii as the cause of urinary tract infection. J Clin Microbiol. 2009 Jan;47(1):275-7. DOI: 10.1128/JCM.01630-08
[17] Lawson PA, Falsen E, Akervall E, Vandamme P, Collins MD. Characterization of some Actinomyces-like isolates from human clinical specimens: reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and description of Actinobaculum schaalii sp. nov. Int J Syst Bacteriol. 1997 Jul;47(3):899-903. DOI: 10.1099/00207713-47-3-899
[18] Lee YS, Kim JY, Kim JC, Park WH, Choo MS, Lee KS. Prevalence and treatment efficacy of genitourinary mycoplasmas in women with overactive bladder symptoms. Korean J Urol. 2010 Sep;51(9):625-30. DOI: 10.4111/kju.2010.51.9.625
[19] Schulte TL. Bacteroides and anaerobic streptococci in infection of the urinary tract. Proc Mayo Clin. 1939;14:536.
[20] Kumazawa J, Kiyohara H, Momose S. Significance of anaerobic bacteria isolated from the urinary tract. II. Experimental studies. Invest Urol. 1976 Jan;13(4):309-12.
[21] Sapico FL, Wideman PA, Finegold SM. Aerobic and anaerobic flora in bladder urine of patients with indwelling urethral catheters. Urology. 1976 Apr;7(4):382-4. DOI: 10.1016/0090-4295(76)90251-X
[22] Brook I. Urinary tract infection caused by anaerobic bacteria in children. Urology. 1980 Dec;16(6):596-8. DOI: 10.1016/0090-4295(80)90566-X
[23] Apostolopoulou C, Konstantoulaki S, Androulakakis P, Vatopoulou T, Varkarakis M. Isolation of anaerobic organisms from kidney in serious renal infections. Urology. 1982 Nov;20(5):479-81. DOI: 10.1016/0090-4295(82)90117-0
[24] Duprey KM, McCrea L, Rabinowitch BL, Azad KN. Pyelonephritis and Bacteremia from Lactobacillus delbrueckii. Case Rep Infect Dis. 2012;2012:745743. DOI: 10.1155/2012/745743
[25] Nabokova IuL, Gudima IA, Kogan MI, Chernitskaia ML. [Bacterial spectrum of the urine in young healthy women]. Urologiia. 2010 Sep-Oct;(5):7-10.
[26] Naboka IuL, Gudima IA, Ibishev KhS, Miroshnichenko EA, Kogan MI, Vasil'eva LI. [Etiological structure and antibiotic sensitivity of uropathogens in chronic recurrent infection of the lower urinary tract]. Urologiia. 2011 Nov-Dec;(6):12-5.
[27] Kogan MI, Naboka YL, Ibishev KS, Gudima IA, Naber KG. Human urine is not sterile - shift of paradigm. Urol Int. 2015;94(4):445-52. DOI: 10.1159/000369631
[28] Kogan MI, Pasechnik DG, Naboka IuL, Ibishev KhS, Gazaev ZI, Gudima IA. [Non-clostridial anaerobic bacteria can cause acute pyelonephritis (an experimental trial)]. Urologiia. 2012 Mar-Apr;(2):8-13.
[29] Naboka IuL, Kogan MI, Gudima IA, Tchernitskaia ML, Ibishev KhS, Khasigov AV, Mitusova EV. [Role of nonclostridial anaerobes in the development of infectious and inflammatory diseases of the urinary and reproductive systems]. Urologiia. 2013 Nov-Dec;(6):118-21.
[30] Naboka IuL, Kogan MI, Vasil'eva LI, Gudima IA, Miroshnichenko EA, Ibishev KhS. [Bacterial mixed infection in women with chronic recurrent cystitis]. Zh Mikrobiol Epidemiol Immunobiol. 2011 Jan-Feb;(1):8-12.
[31] Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, Kliethermes S, Schreckenberger PC, Brubaker L, Gai X, Wolfe AJ. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014 Jul;5(4):e01283-14. DOI: 10.1128/mBio.01283-14
[32] Wolfe AJ, Brubaker L. "Sterile Urine" and the Presence of Bacteria. Eur Urol. 2015 Aug;68(2):173-4. DOI: 10.1016/j.eururo.2015.02.041
[33] Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, Mueller ER, Schreckenberger P, Dong Q, Nelson DE, Brubaker L. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012 Apr;50(4):1376-83. DOI: 10.1128/JCM.05852-11
[34] Groah S, Perez-Losada M, Caldovic L, Ljungberg I, Sprague B, Castro-Nallar E, Shah N, Hsieh M, Pohl H. Pyuria and asymptomatic bacteriuria is associated with novel and specific urine microbiomes. J Urol. 2015;193(4):e226. DOI: 10.1016/j.juro.2015.02.980
[35] Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, Sodergren E, Weinstock GM, Diao L, Fortenberry JD. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS ONE. 2010 Nov;5(11):e14116. DOI: 10.1371/journal.pone.0014116
[36] Nickel JC, Stephens A, Chen J, Melton-Kreft R, Spirk T, Earl J, O'Toole M, Costerton JW, vanBokhoven A, Mullins C, Ehrlich G. Application of state-of-the-art methods to search for microbial contributions to the etiology of urological chronic pelvic pain syndrome (UCPPS). J Urol. 2013;189(4):e468-9. DOI: 10.1016/j.juro.2013.02.782
[37] Dong Q, Nelson DE, Toh E, Diao L, Gao X, Fortenberry JD, Van der Pol B. The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens. PLoS ONE. 2011;6(5):e19709. DOI: 10.1371/journal.pone.0019709
[38] Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013 Nov;369(20):1883-91. DOI: 10.1056/NEJMoa1302186
[39] Imirzalioglu C, Hain T, Chakraborty T, Domann E. Hidden pathogens uncovered: metagenomic analysis of urinary tract infections. Andrologia. 2008 Apr;40(2):66-71. DOI: 10.1111/j.1439-0272.2007.00830.x
[40] Khasriya R, Sathiananthamoorthy S, Ismail S, Kelsey M, Wilson M, Rohn JL, Malone-Lee J. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol. 2013 Jul;51(7):2054-62. DOI: 10.1128/JCM.03314-12
[41] Aagaard K, Luna R.A, Versalovic J. The Human Microbiome of Local Body Sites and Their Unique Biology. In: Mandell GL, editor. Principles and Practice of infectious Diseases. 8th ed. London: Elsevier; 2015. p. 11-18.
[42] Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011 Nov;11:244. DOI: 10.1186/1471-2180-11-244
[43] Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, Huang ST, Ljungberg I, Sprague BM, Lucas SK, Torralba M, Nelson KE, Groah SL. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012 Aug;10:174. DOI: 10.1186/1479-5876-10-174
[44] Naboka YL, Kogan MI, Mitusova EV, Gudima IA. Microbiota bladder and pelvis urine in complicated infections of the upper urinary tract. European Urology Supplements. 2015;14(2): e259-e259a. DOI: 10.1016/S1569-9056(15)60257-3
[45] Giamarellos-Bourboulis EJ, Adamis T, Laoutaris G, Sabracos L, Koussoulas V, Mouktaroudi M, Perrea D, Karayannacos PE, Giamarellou H. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004 Jan;48(1):93-9.
[46] Zeidan S, El Ghoneimi A, Peuchmaur M, Bingen E, Bonacorsi S. Effect of partial ureteral obstruction and bacterial virulence on the occurrence of renal scarring in a mouse model. Urology. 2012 Aug;80(2):486.e1-7. DOI: 10.1016/j.urology.2012.02.020
[47] Pasechnik DG, Kogan MI, Naboka JL, Ibishev H, Gazaev Z, Gudima I. The experimental model of obstructive acute pyelonephritis induced non-clostridial infection. Plenary Oral Free Paper Sessions. Virchows Archiv. 2011 Aug;459(S1):1–329. DOI: 10.1007/s00428-011-1113-y
[48] Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982 Aug;307(8):463-8. DOI: 10.1056/NEJM198208193070802
[49] Saginur R, Nicolle LE. Single-dose compared with 3-day norfloxacin treatment of uncomplicated urinary tract infection in women. Canadian Infectious Diseases Society Clinical Trials Study Group. Arch Intern Med. 1992 Jun;152(6):1233-7. DOI: 10.1001/archinte.1992.00400180091014
[50] Nicolle LE, DuBois J, Martel AY, Harding GK, Shafran SD, Conly JM. Treatment of acute uncomplicated urinary tract infections with 3 days of lomefloxacin compared with treatment with 3 days of norfloxacin. Antimicrob Agents Chemother. 1993 Mar;37(3):574-9. DOI: 10.1128/AAC.37.3.574
[51] Grabe M, Bartoletti R, Bjerklund Johansen TE, Cai T, Çek M, Köves B, Naber KG, Pickard RS, Tenke P, Wagenlehner F, Wullt B; European Association of Urology. Guidelines on Urological Infections. Arnhem. European Association of Urology; 2015. Available from: http://uroweb.org/wp-content/uploads/EAU-Guidelines-Urological-Infections-v2.pdf
[52] Kass EH. Chemotherapeutic and antibiotic drugs in the management of infections of the urinary tract. Am J Med. 1955 May;18(5):764-81. DOI: 10.1016/0002-9343(55)90190-X




