Integration of a medication reminder app into the RABBIT-SpA disease registry to record patient data on daily drug usage
Anne C. Regierer 1Andreas Reich 1
Anja Weiß 1
Martin Feuchtenberger 2
Silke Zinke 3
Hanns-Martin Lorenz 4
Uta Syrbe 5
Peter Böhm 6
Julius Wiegand 7
Anja Strangfeld 1,8
Denis Poddubnyy 9
Xenofon Baraliakos 10
1 German Rheumatology Research Center (DRFZ Berlin), Epidemiology and Health Services Research, Berlin, Germany
2 Department of Internal Medicine II, InnKlinikum, Burghausen, Germany
3 Rheumatologische Schwerpunktpraxis Dr. med. Silke Zinke, Berlin, Germany
4 Department of Internal Medicine V, Heidelberg University Hospital, Heidelberg, Germany
5 Rheumatologische Schwerpunktpraxis Rheuma Praxis Berlin, Berlin, Germany
6 Deutsche Rheuma-Liga Bundesverband, Berlin, Germany
7 Rheuma-Liga Baden-Württemberg e.V., Heidelberg, Germany
8 Charité – Universitätsmedizin Berlin, Berlin, Germany
9 Charité – Universitätsmedizin Berlin, Department of Gastroenterology, Infectiology and Rheumatology, Berlin, Germany
10 Rheumazentrum Ruhrgebiet, Marien Hospital Herne – University Hospital Ruhr University Bochum, Herne, Germany
Abstract
Background: The main goals of the disease registry RABBIT-SpA for axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) are to analyse the long-term effectiveness and safety of the antirheumatic drugs used for these chronic conditions. While the registry focuses on documenting antirheumatic drugs, the recording of co-medication has been inadequate, despite growing awareness of the importance of comorbidities in the outcomes of rheumatic and musculoskeletal diseases.
Research question and objective: The primary objective is the data protection-compliant integration of a medication reminder app into the registry’s existing online documentation system, providing a technically simple solution for participating rheumatologists. Secondary objectives include the direct documentation of medication by patients using the integrated app and the more frequent capture of patient-reported outcome (PRO) parameters. The main research question of this preliminary analysis is the concordance between the anti-rheumatic medication documented by the patient via the app and by the rheumatologist via the registry.
Methods: RABBIT-SpA is a disease registry for axSpA and PsA. A study version of the MyTherapy app was developed by expanding the generic app, which primarily records medication and provides medication reminders, to include questions from the RABBIT-SpA patient questionnaire (covering pain, health status, global disease activity, sleep disorders, and skin involvement). Patients use their own Android or iOS smartphones and provide informed consent for participation.
Results: The integration process was successfully implemented. Personalized QR codes were generated from the registry documentation system, enabling patients to download the study version of the MyTherapy app and link their data using a second pseudonym. As of now, 367 patients have been invited to participate, with 139 consenting and 76 successfully using the app. App users are generally younger and more often male compared to the overall registry cohorts. On average, 4.7 medications were recorded per patient (range 1–42), with a high concordance (94%) between the medications documented in the app and those in the registry. The proportion of patients with polypharmacy (defined as the simultaneous documentation of five or more medications) is 25%.
Discussion: The integration of the medication reminder app into the RABBIT-SpA registry was successfully achieved in a data protection-compliant manner. The antirheumatic drugs documented in the app were concordant to the registry data. The integration of an app to an ongoing disease registry offers the possibility of supplementing data from the registry with patient generated data.
Keywords
disease registry, spondyloarthritis, medical smartphone app, data linkage, patient-reported outcomes
Introduction
The main objectives of the RABBIT-SpA disease registry, initiated by the German Rheumatism Research Center Berlin (DRFZ) in 2017, are to investigate the long-term effectiveness and safety of drug therapies for axial spondyloarthritis (axSpA; ankylosing spondylitis) and psoriatic arthritis (PsA) [1]. Both diseases cause significant pain and functional limitations, impacting patients’ quality of life. Since the early 2000s, various innovative drugs, mainly biologic disease-modifying anti-rheumatic drugs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs), have been approved in Germany, revolutionizing therapy and positively altering disease progression. The primary representatives of these b/tsDMARDs are cytokine inhibitors, such as tumor necrosis factor alpha inhibitors (TNFi), interleukin (IL) inhibitors (IL-17i and IL12/23-i), and Janus kinase inhibitors (JAKi). Despite their efficacy in clinical trials, these DMARDs are only effective in approximately 60–70% of patients, with secondary effectiveness failures. Increased infection risk is the most relevant adverse event in these immunomodulating substances [2], [3].
AxSpA and PsA are associated with other chronic diseases, particularly metabolic syndrome in PsA, which significantly increases cardiovascular risk and mortality [4]. The interactions between the disease activity of chronic inflammatory conditions and comorbidities like coronary heart disease (CHD) are not fully understood. However, it is known that prolonged high disease activity increases cardiovascular mortality [5], [6]. A lack of therapy for comorbid conditions increased mortality even more as shown in an analysis of the German Biologics Registry for Rheumatoid Arthritis (RABBIT Registry) [6]. In contrast to the comprehensive documentation of the anti-rheumatic treatment, the treatment of comorbidities is incompletely recorded in the RABBIT-SpA registry, as the focus lies on the treatment of the underlying chronic inflammatory disease. This is partly due to the fact that the rheumatologist might not always have all relevant information from the specialist disciplines for multimorbid patients.
Due to demographic changes, the proportion of people with multiple chronic diseases is increasing in Germany and many other countries [7]. Multiple chronic diseases can lead to polypharmacy. The definition of polypharmacy is not standardized, often the daily intake of 5 or more medications is used as a definition [8]. Polypharmacy can lead to adverse drug reactions and drug interactions. In Germany, 25% of people across all age groups are affected by polypharmacy, and this proportion rises to 44% in those aged 65 years and older [9]. Despite the increasing focus on the influence of comorbidities and multimorbidity in rheumatic diseases, there is limited data on the frequency of polypharmacy in these patients [10].
People with multiple chronic conditions leading to polypharmacy might experience difficulties in adhering to the daily medication. Medication reminder apps have been developed to assist patients with their daily medication regimen and might be especially helpful for people with multiple daily medications. There are a number of generic freely available apps that are widely used. The MyTherapy app allows for user-friendly medication entry, either by scanning the barcode or selecting the medication from a list. Its reminder function is designed to increase medication adherence [11].
Patient-reported outcomes (PROs) are well-established in rheumatology and are part of standard care. According to clinical guidelines, PROs are used for shared treatment decision making in addition to results from clinical examinations (especially joint function) and laboratory parameters [12], [13]. Traditionally, PROs are collected via paper or tablet questionnaires during visits to the rheumatologist, but smartphone or desktop-based apps are increasingly utilized. There are various approaches to using innovative digital tools to improve care. However, it remains uncertain whether recording data via an app, which can be done more frequently, offers an advantage for patients and rheumatologists.
Medical smartphone apps, as well as data from sensors or trackers, offer the potential to generate valuable patient-reported information. Integrating this patient data into well-monitored longitudinal registry data [14] might enhance completeness of information and enable new analyses. Therefore, in this BMBF-funded project, a medication reminder app was modified for the RABBIT-SpA registry, and a data protection-compliant linkage process was established. The aim is to collect medication details directly from patients using the mobile app. Besides a description of the integration of the app into the registry the main research question of this analysis is the concordance between the anti-rheumatic medication documented by the patient via the app and by the rheumatologist via the registry documentation.
Methods
RABBIT-SpA is a disease registry with the study design of a longitudinal epidemiological observational cohort study. Adult patients with confirmed diagnoses of axSpA or PsA starting a new drug treatment can be included. Patients are observed over 10 years. Both physician and patient questionnaires are administered at regular intervals via a browser-based documentation system. Questionnaires are completed at inclusion, after 3 months, after 6 months, and every 6 months thereafter. Various processes automatically check for plausibility and completeness [14].
Established instruments are used in the RABBIT-SpA patient questionnaires to capture the various aspects of these complex diseases. Besides the disease-specific tools commonly employed in rheumatology to measure disease activity, function, and quality of life, the questionnaires also inquire about the patient’s occupational situation and ability to work.
Patients can participate in the project using their own Android or iOS smartphones. They are informed about the project and in addition to consenting to RABBIT-SpA, they sign an informed consent for the additional app project.
The generic and freely available MyTherapy app by smartpatient was selected for the project. In a participatory approach, together with research partners from the German Rheumatism League, specific questions from the RABBIT-SpA patient questionnaire were selected. In two workshops and in biweekly video-calls, the patient questionnaire from RABBIT-SpA was analysed. A list was developed identifying the most relevant disease domains captured by the PRO instruments. Then, the instruments were evaluated for the feasibility to include them into the app. The instruments were selected based on mutual agreement between the research partners. They focus on health status, disease activity, pain, sleep disorders, and, for PsA, skin involvement (Figure 1 [Fig. 1], all using a numerical rating scale [0–10]). Patients receive weekly push notifications to answer these questions, which can also be completed proactively at any time.
Figure 1: Excerpt from the patient questionnaire from RABBIT-SpA. The five questions are assessing state of health, disease activity, pain, skin involvement, and sleep disorders using a numerical rating scale [0–10].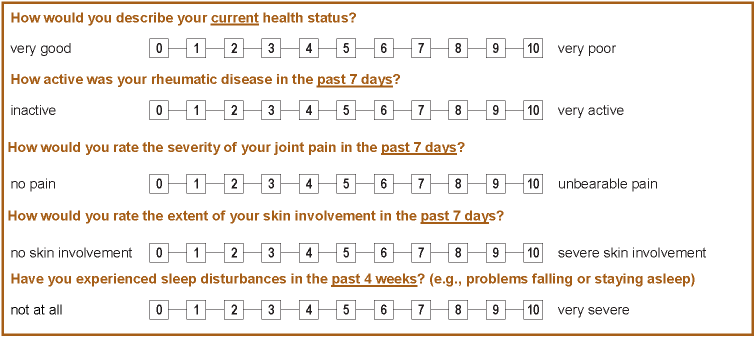
To enhance user-friendliness, study-specific information texts were added to the app, covering topics like DRFZ disease registers, patient organizations, and the research project’s objectives [15], [16]. The app also includes a motivational feature called “photo of the day”, displayed once all medication reminders are processed for the day.
For this preliminary analysis, medications documented in the app from Anatomical Therapeutic Chemical (ATC) groups L (antineoplastic and immunomodulatory substances) and M (musculoskeletal system) were systematically compared with the registry data.
The ethics committee of Charité Universitätsmedizin Berlin granted ethics approval for the app’s integration into the registry on June 24, 2021 (EA1/246/16).
Results
Description of the process
All RABBIT-SpA registry patients can participate in the app project. After providing informed consent, a personalized QR code is generated from the registry’s documentation system. This QR code contains a project pseudonym that can be linked to the registry pseudonym in the documentation system. The QR code also contains the link to download the app (Android and iOS) and the specific modification of the app, which is configured differently for axSpA and PsA.
By scanning the personalized QR code, patients are directed to the study version of the app in the corresponding app store. After agreeing to the terms of use and privacy policy, the project pseudonym is automatically saved in the app and patients can use the app immediately. Study centers encourage patients to scan the QR code immediately to ensure the app is ready for use.
Data flow is illustrated in Figure 2 [Fig. 2]. The RABBIT-SpA documentation system tracks patients’ consent or refusal to participate in the app project. A list of project pseudonyms of consenting patients can be exported from the registry documentation system.
Figure 2: Pictogram of the data flow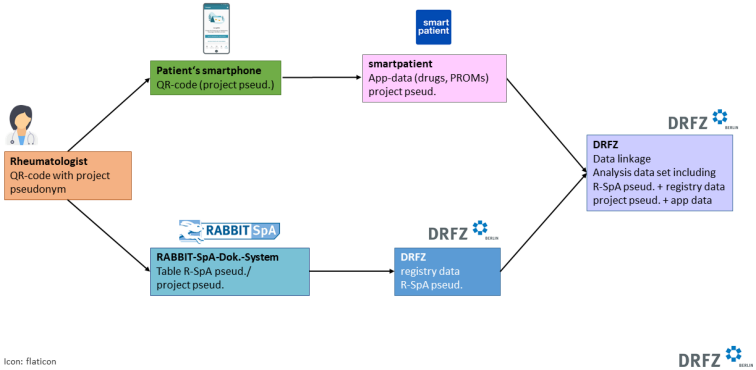
App data is stored on smartpatient’s protected server. The study participants’ data is downloaded from the DRFZ once a week as part of a protected data export and stored on a protected DRFZ server. The registry data and the app data can be linked via the registry and project pseudonym and an analysis data set can be created.
An example of the linked data of a patient is shown graphically in Figure 3 [Fig. 3].
Figure 3: Example of the linked data of one patient. The red dots show the state of health (NRS 0-10) recorded by the patient directly in the app. The blue dots show the state of health (NRS 0-10) recorded by the patient in the registry documentation system at fixed time intervals. The blue and green arrow symbolize the planned registry visit time intervals at which patient and physicians document into the registry documentation system.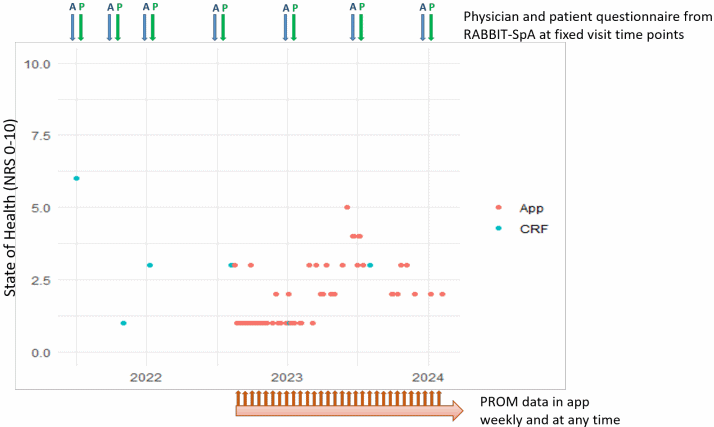
Preliminary data analysis
As of now, 367 RABBIT-SpA patients have been invited to participate in the app project, with 139 consenting and receiving a QR code, and 76 successfully installing and using the app. Table 1 [Tab. 1] compares the characteristics of app users to the overall registry cohort, highlighting known differences between axSpA and PsA patients. For example, axSpA patients are younger and more often men compared to PsA patients. App users tend to be younger and more often male in both axSpA and PsA patients (Table 1 [Tab. 1]).
Table 1: Characteristics of the app users compared to the other RABBIT-SpA patients (registry data set)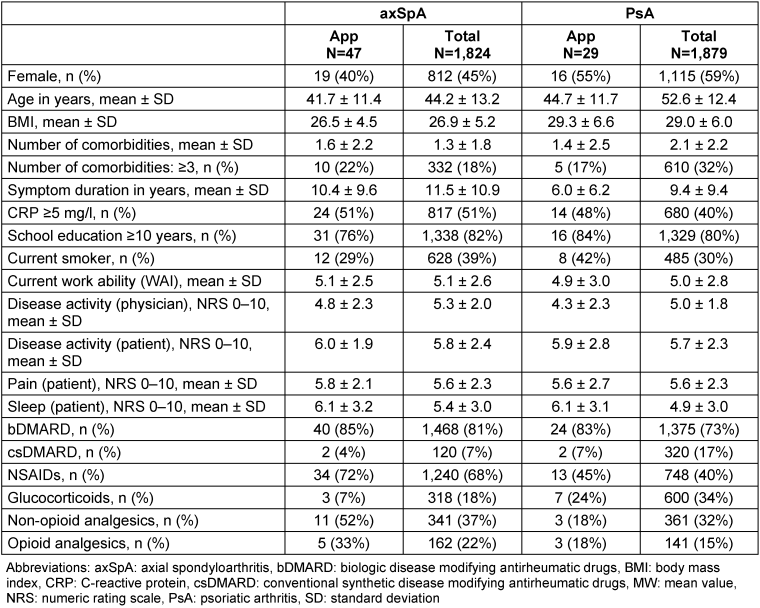
The proportion of PsA patients with ≥3 comorbidities is lower in the app users than in the overall PsA cohort. The patient-reported parameters from the registry data set at inclusion into the registry, in particular pain and global disease activity, are very similar between the app users and the overall cohort for both diseases.
App users actively used the app for an average of 97 days, with 74% continuing use after one month and 53% after three months.
Medication data from the app is detailed in Table 2 [Tab. 2] and Table 3 [Tab. 3]. Most frequently recorded medications belong to ATC groups L, which includes immunomodulatory drugs, and M which includes non-steroidal anti-inflammatory drugs (NSAIDs).
Table 2: Medication by ATC group from the app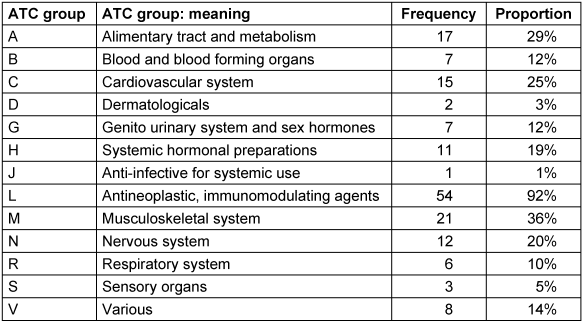
Table 3: Most common medications in the app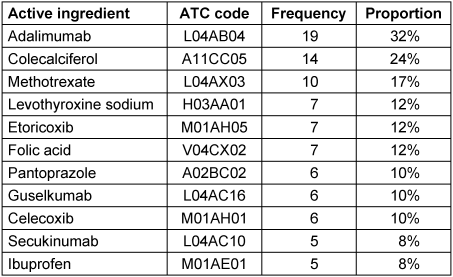
In order to analyze the concordance between the anti-rheumatic medication documented by the patient via the app and by the rheumatologist via the registry drugs from ATC groups L and M were systematically compared with the treatments recorded on the physician’s questionnaire from the registry at the appropriate time point. There was high concordance between app and registry documentation. Of 54 patients, 69 DMARDs were documented in the app. 65 (94%) of these substances were also documented in the registry questionnaire. 22 different substances from ATC group M were documented in the app by 21 patients. Of these, 20 (91%) were also documented in the registry questionnaire.
On average, 4.7 (range 1–42) medications were recorded per patient in the app, with axSpA patients averaging 3.6 medications and PsA patients 5.1 medications. The proportion of patients with polypharmacy (defined as the simultaneous documentation of five or more medications) is 25%. Patients with polypharmacy (n=13) had an average of 2.6 comorbidities, while those without polypharmacy (n=40) had an average of 1.3 comorbidities. Additionally, PROs were significantly worse in the polypharmacy group, with an average pain score of 6.5 compared to 5.1 in those without polypharmacy. Notably, 6 out of 13 polypharmacy patients received systemic glucocorticoids, compared to just 3 out of 40 in the non-polypharmacy group.
Discussion
Medical smartphone apps can be used to generate PROs, which can significantly promote patients’ participation in their own healthcare. Integrating these apps into ongoing disease registries provides an opportunity to enrich registry data with additional patient-generated data. In this project, the integration of a medication reminder app into the RABBIT-SpA registry was successfully implemented. We found a high concordance between the anti-rheumatic medication documented by the patient via the app and by the rheumatologist via the registry.
Clinical trials remain the gold standard for analyzing the efficacy of new treatments. The strengths of clinical trials include randomization and blinding, which allow for a direct analysis of the effects of study interventions. However, clinical trials often suffer from low external validity due to their narrow inclusion and exclusion criteria, short study durations that do not allow for the investigation of long-term effects (both effectiveness and safety), and small group sizes that do not permit the analysis of very rare adverse events [17]. In this context, observational studies and data from medical registries provide valuable complementary evidence [18]. The relevance of registry data has increased in recent years, as data from well-monitored, high-quality registries are increasingly being used to analyze treatment safety and effectiveness in unselected patient cohorts. Enhancing the quality of registry data is crucial so that these valuable data can be effectively utilized [19].
In rheumatology, disease registries are highly accepted, and many clinically relevant findings have been published from the RABBIT and RABBIT-SpA registries [20].
One way to further improve the usability of registry data is by linking it with other data sources [21]. In our research project, a smartphone app was linked to the disease registry as part of a pilot project. Mobile health technologies like smartphone apps are rapidly evolving and have the potential to improve medical care especially as a shortage of health care professionals is an increasing problem [22], [23]. The MyTherapy app was chosen because it is a generic, freely available app that allows for user-friendly medication entry, either by scanning the barcode or selecting the medication from a list. We developed and implemented a data protection-compliant process that is quick and easy for both participating physicians and patients. This process was designed to be flexible, allowing for the future integration of other apps into the registry.
The MyTherapy app aims to assist patients with their daily medication regimen. Its reminder function is designed to increase medication adherence [11]. Another feature of the app helps users to monitor their medication supply, reminding them to consider follow-up prescriptions for their long-term medications. The usage behavior of app users in our project is comparable to that of users of other medical smartphone apps [15], [24], [25]. Users of the generic version of the MyTherapy app typically enter an average of four medications (unpublished data, smartpatient), a range similar to that observed in our project.
The preliminary analysis of the medication data collected from the app showed interesting results. The concordance with the physician reported medication data in the registry was very high. However, we only analyzed the concordance of the antirheumatic drugs, due to the low number of participating patients.
Due to demographic changes, the proportion of people with multiple chronic diseases is increasing in Germany and many other countries. Multimorbidity and increasing age are often associated with polypharmacy. While general population data in Germany show that 25% of people across all age groups are affected by polypharmacy, and 44% in those aged 65 years and older, there is only very limited data on the frequency of polypharmacy in people with rheumatic diseases including PsA. In a secondary data analysis of claims data, we found that 49% of PsA patients were prescribed five or more medications, compared to 17% in an age- and gender-matched comparison group without chronic inflammatory rheumatic disease [26]. The influence of age and comorbidities on the number of medications was confirmed in this data analysis. The analysis of the medication data collected from the app in regard to polypharmacy aligns with the expected magnitude. Among patients with an average age of 44 years, the proportion of polypharmacy was 25%, compared to 31% in the same age group according to the Barmer analysis [26]. Additionally, in the registry data, the number of medications correlated with both age and the number of comorbidities.
The collection of PROs is highly relevant in rheumatology, as many PROs are used in treatment decision-making. Medical smartphone apps offer the potential to collect PROs more frequently and outside of clinical settings, such as at home or at work. This easier and more frequent collection of PRO data can enhance patient participation in treatment decisions [27]. The equivalence of data from PROs recorded either on paper or using an app has been shown in a pilot study of 69 axSpA patients, however the adherence to use the app was lower than 30% after 3 and 6 months [28]. In our preliminary analysis we found that around 50% of the patients were still actively using the app after 6 months. In a randomized controlled trial testing different digital follow-up strategies a high degree of acceptance and adherence to reporting PROs using an app has been shown [29]. In a systematic review summarizing the evidence on patients’ engagement with different digital health applications in chronic arthritis a high level of engagement to e-health applications was found [30]. However, it needs to be further elucidated which factors influence the engagement to use medical apps.
Our analysis has limitations. The most prominent limitation is the low number of patients. In Germany, there is an increasing shortage of rheumatologists leading to rising numbers of patients per rheumatologists [22]. The high burden of work load in daily clinical rheumatology care might partially explain the low recruitment into this research project.
Due to the low number of patients, we only analyzed the concordance of the most frequent medication groups documented in the app, which were the antirheumatic drugs. However, it would be of interest to analyze the concordance between the app and the registry data on medication for comorbidities as well.
Conclusion
Medical registries can be improved by linking app-generated data. The availability of linked data offers opportunities to answer a variety of new research questions. The integration of medical apps offers a promising avenue for greater patient participation and engagement in their own healthcare.
Notes
Authors’ ORCIDs
- Anne C. Regierer: 0000-0003-2456-4049
- Andreas Reich: 0000-0002-3729-0772
- Anja Weiß: 0000-0003-2069-2190
- Martin Feuchtenberger: 0000-0001-8028-8099
- Hanns-Martin Lorenz: 0000-0001-8197-8681
- Uta Syrbe: 0000-0001-8463-7890
- Peter Böhm: 0000-0002-7186-1997
- Anja Strangfeld: 0000-0002-6233-022X
- Denis Poddubnyy: 0000-0002-4537-6015
- Xenofon Baraliakos: 0000-0002-9475-9362
Competing interests
The authors declare that they have no competing interests.
References
[1] Regierer AC, Weiß A, Baraliakos X, Zink A, Listing J, Strangfeld A. RABBIT-SpA: ein neues Krankheitsregister für axiale Spondyloarthritis und Psoriasisarthritis [RABBIT-SpA: a new disease register for axial spondyloarthritis and psoriatic arthritis]. Z Rheumatol. 2020 Mar;79(2):135-42. DOI: 10.1007/s00393-019-0613-z[2] Leung YY, Korotaeva TV, Candia L, Pedersen SJ, Bautista Molano W, Ruderman EM, Bisoendial R, Perez-Alamino R, Olsder W, Möller B, Grazio S, Gudu T, Mody GM, Pineda C, Raffayova H, Rohekar S, Goldenstein-Schainberg C, Gutierrez Urena SR, Casasola Vargas JC, Meghnathi B, Prasad R, Richette P, Miranda JRS, Malliotis N, Lindqvist U, Simon D, Ezeonyeji A, Soriano ER, FitzGerald O. Management of Peripheral Arthritis in Patients With Psoriatic Arthritis: An Updated Literature Review Informing the 2021 GRAPPA Treatment Recommendations. J Rheumatol. 2023 Jan;50(1):119-30. DOI: 10.3899/jrheum.220315
[3] Webers C, Ortolan A, Sepriano A, Falzon L, Baraliakos X, Landewé RBM, Ramiro S, van der Heijde D, Nikiphorou E. Efficacy and safety of biological DMARDs: a systematic literature review informing the 2022 update of the ASAS-EULAR recommendations for the management of axial spondyloarthritis. Ann Rheum Dis. 2023 Jan;82(1):130-41. DOI: 10.1136/ard-2022-223298
[4] Exarchou S, Di Giuseppe D, Klingberg E, Sigurdardottir V, Wedrén S, Lindström U, Turesson C, Jacobsson LTH, Askling J, Wallman JK. Mortality in patients with psoriatic arthritis in Sweden: a nationwide, population-based cohort study. Ann Rheum Dis. 2024 Mar;83(4):446-56. DOI: 10.1136/ard-2023-224965
[5] Meissner Y, Richter A, Manger B, Tony HP, Wilden E, Listing J, Zink A, Strangfeld A. Serious adverse events and the risk of stroke in patients with rheumatoid arthritis: results from the German RABBIT cohort. Ann Rheum Dis. 2017 Sep;76(9):1583-90. DOI: 10.1136/annrheumdis-2017-211209
[6] Meissner Y, Zink A, Kekow J, Rockwitz K, Liebhaber A, Zinke S, Gerhold K, Richter A, Listing J, Strangfeld A. Impact of disease activity and treatment of comorbidities on the risk of myocardial infarction in rheumatoid arthritis. Arthritis Res Ther. 2016 Aug;18(1):183. DOI: 10.1186/s13075-016-1077-z
[7] Buehring B, van Onna M, Myasoedova E, Lee J, Makris UE. Understanding the multiple dimensions of ageing: 5Ms for the rheumatologist. Lancet Rheumatol. 2024 Dec;6(12):e892-e902. DOI: 10.1016/S2665-9913(24)00230-3
[8] Marengoni A, Onder G. Guidelines, polypharmacy, and drug-drug interactions in patients with multimorbidity. BMJ. 2015 Mar;350:h1059. DOI: 10.1136/bmj.h1059
[9] Grandt D, Lappe V, Schubert I. Barmer Arzneimittelreport 2020. Sektorenübergreifende Arzneimitteltherapie. Berlin: Barmer; 2020. (Schriftenreihe zur Gesundheitsanalyse; 23).
[10] Bechman K, Clarke BD, Rutherford AI, Yates M, Nikiphorou E, Molokhia M, Norton S, Cope AP, Hyrich KL, Galloway JB. Polypharmacy is associated with treatment response and serious adverse events: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 2019 Oct;58(10):1767-76. DOI: 10.1093/rheumatology/kez037
[11] Al Dameery K, Valsaraj BP, Qutishat M, Obeidat A, Alkhawaldeh A, Al Sabei S, Al Omari O, ALBashtawy M, Al Qadire M. Enhancing Medication Adherence Among Patients With Schizophrenia and Schizoaffective Disorder: Mobile App Intervention Study. SAGE Open Nurs. 2023;9:23779608231197269. DOI: 10.1177/23779608231197269
[12] Coates LC, Soriano ER, Corp N, Bertheussen H, Callis Duffin K, Campanholo CB, Chau J, Eder L, Fernández-Ávila DG, FitzGerald O, Garg A, Gladman DD, Goel N, Helliwell PS, Husni ME, Jadon DR, Katz A, Laheru D, Latella J, Leung YY, Lindsay C, Lubrano E, Mazzuoccolo LD, Mease PJ, O'Sullivan D, Ogdie A, Olsder W, Palominos PE, Schick L, Steinkoenig I, de Wit M, van der Windt DA, Kavanaugh A; GRAPPA Treatment Recommendations domain subcommittees. Author Correction: Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022 Dec;18(12):734. DOI: 10.1038/s41584-022-00861-w
[13] Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, Landewé RBM, Van den Bosch FE, Boteva B, Bremander A, Carron P, Ciurea A, van Gaalen FA, Géher P, Gensler L, Hermann J, de Hooge M, Husakova M, Kiltz U, López-Medina C, Machado PM, Marzo-Ortega H, Molto A, Navarro-Compán V, Nissen MJ, Pimentel-Santos FM, Poddubnyy D, Proft F, Rudwaleit M, Telkman M, Zhao SS, Ziade N, van der Heijde D. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. DOI: 10.1136/ard-2022-223296
[14] Lindner L, Weiß A, Reich A, Kindler S, Behrens F, Braun J, Listing J, Schett G, Sieper J, Strangfeld A, Regierer AC. Implementing an automated monitoring process in a digital, longitudinal observational cohort study. Arthritis Res Ther. 2021 Jul;23(1):181. DOI: 10.1186/s13075-021-02563-2
[15] Knitza J, Simon D, Lambrecht A, Raab C, Tascilar K, Hagen M, Kleyer A, Bayat S, Derungs A, Amft O, Schett G, Hueber AJ. Mobile Health Usage, Preferences, Barriers, and eHealth Literacy in Rheumatology: Patient Survey Study. JMIR Mhealth Uhealth. 2020 Aug;8(8):e19661. DOI: 10.2196/19661
[16] Tran S, Smith L, Carter S. Understanding Patient Perspectives on the Use of Gamification and Incentives in mHealth Apps to Improve Medication Adherence: Qualitative Study. JMIR Mhealth Uhealth. 2024 May;12:e50851. DOI: 10.2196/50851
[17] Zink A, Strangfeld A, Schneider M, Herzer P, Hierse F, Stoyanova-Scholz M, Wassenberg S, Kapelle A, Listing J. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006 Nov;54(11):3399-407. DOI: 10.1002/art.22193
[18] Franklin JM, Pawar A, Martin D, Glynn RJ, Levenson M, Temple R, Schneeweiss S. Nonrandomized Real-World Evidence to Support Regulatory Decision Making: Process for a Randomized Trial Replication Project. Clin Pharmacol Ther. 2020 Apr;107(4):817-26. DOI: 10.1002/cpt.1633
[19] Stausberg J, Maier B, Bestehorn K, Gothe H, Groene O, Jacke C, Jänicke M, Kostuj T, Mathes T, Niemeyer A, Olbrich K, Schmitt J, Neugebauer E. Memorandum Register für die Versorgungsforschung: Update 2019 [Memorandum Registry for Health Services Research: Update 2019]. Gesundheitswesen. 2020 Mar;82(3):e39-e66. DOI: 10.1055/a-1083-6417
[20] Strangfeld A, Albrecht K, Regierer A, Callhoff J, Zink A, Minden K. 33 Jahre DRFZ: Epidemiologie und Versorgungsforschung [Celebrating 33 years of the DRFZ: Epidemiology and Health Services Research]. Z Rheumatol. 2022 Oct;81(8):642-51. DOI: 10.1007/s00393-022-01187-4
[21] Stubbs E, Exley J, Wittenberg R, Mays N. How to establish and sustain a disease registry: insights from a qualitative study of six disease registries in the UK. BMC Med Inform Decis Mak. 2024 Nov;24(1):361. DOI: 10.1186/s12911-024-02775-x
[22] Braun J, Albrecht K, Callhoff J, Haase I, Krause A, Lakomek HJ, Meyer-Olson D, Schmale-Grede R, Wagner U, Zeidler J, Zinke S, Voormann A, Specker C; die Kommission Versorgung der DGRh. Rheumatologische Versorgung in Deutschland: Memorandum der Deutschen Gesellschaft für Rheumatologie und Klinische Immunologie 2024 [Rheumatological care in Germany: Memorandum of the German Society for Rheumatology and Clinical Immunology 2024]. Z Rheumatol. 2024 Aug;83(Suppl 2):249-84. DOI: 10.1007/s00393-024-01539-2
[23] Najm A, Nikiphorou E, Kostine M, Richez C, Pauling JD, Finckh A, Ritschl V, Prior Y, Balážová P, Stones S, Szekanecz Z, Iagnocco A, Ramiro S, Sivera F, Dougados M, Carmona L, Burmester G, Wiek D, Gossec L, Berenbaum F. EULAR points to consider for the development, evaluation and implementation of mobile health applications aiding self-management in people living with rheumatic and musculoskeletal diseases. RMD Open. 2019;5(2):e001014. DOI: 10.1136/rmdopen-2019-001014
[24] Najm A, Lempp H, Gossec L, Berenbaum F, Nikiphorou E. Needs, Experiences, and Views of People With Rheumatic and Musculoskeletal Diseases on Self-Management Mobile Health Apps: Mixed Methods Study. JMIR Mhealth Uhealth. 2020 Apr;8(4):e14351. DOI: 10.2196/14351
[25] Colls J, Lee YC, Xu C, Corrigan C, Lu F, Marquez-Grap G, Murray M, Suh DH, Solomon DH. Patient adherence with a smartphone app for patient-reported outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2021 Jan;60(1):108-12. DOI: 10.1093/rheumatology/keaa202
[26] Albrecht K, Regierer AC, Strangfeld A, Marschall U, Callhoff J. High burden of polypharmacy and comorbidity in persons with psoriatic arthritis: an analysis of claims data, stratified by age and sex. RMD Open. 2023 Mar;9(1):e002960. DOI: 10.1136/rmdopen-2022-002960
[27] Labinsky H, May S, Boy K, von Rohr S, Grahammer M, Kuhn S, Rojas-Restrepo J, Vogt E, Heinze M, Schett G, Muehlensiepen F, Knitza J. Evaluation of a hybrid telehealth care pathway for patients with axial spondyloarthritis including self-sampling at home: results of a longitudinal proof-of-concept mixed-methods study (TeleSpactive). Rheumatol Int. 2024 Jun;44(6):1133-42. DOI: 10.1007/s00296-024-05581-w
[28] Kempin R, Richter JG, Schlegel A, Baraliakos X, Tsiami S, Buehring B, Kiefer D, Braun J, Kiltz U. Monitoring of Disease Activity With a Smartphone App in Routine Clinical Care in Patients With Axial Spondyloarthritis. J Rheumatol. 2022 Aug;49(8):878-84. DOI: 10.3899/jrheum.211116
[29] Thomassen EEK, Berg IJ, Kristianslund EK, Tveter AT, Bakland G, Gossec L, Hakim S, Macfarlane GJ, de Thurah A, Østerås N. Patients with axial spondyloarthritis reported willingness to use remote care and showed high adherence to electronic patient-reported outcome measures: an 18-month observational study. Rheumatol Int. 2024 Oct;44(10):2089-98. DOI: 10.1007/s00296-024-05673-7
[30] Doumen M, De Cock D, Van Lierde C, Betrains A, Pazmino S, Bertrand D, Westhovens R, Verschueren P. Engagement and attrition with eHealth tools for remote monitoring in chronic arthritis: a systematic review and meta-analysis. RMD Open. 2022 Oct;8(2):e002625. DOI: 10.1136/rmdopen-2022-002625




