Evaluating lipid-driven insulin resistance via TyG index in breast cancer patients: Toward effective secondary prevention
Bandana Kumari 1Rijhul Lahariya 2
1 Department of Biochemistry, All India Institute of Medical Sciences, Patna, Bihar, India
2 All India Institute of Medical Sciences, Patna, Bihar, India
Abstract
Objective: Breast cancer is the most commonly diagnosed malignancy worldwide. Insulin resistance (IR) plays a key role in its progression by activating oncogenic signaling pathways. The triglyceride-glucose (TyG) index is a validated, cost-effective surrogate marker for IR. This study aims to evaluate the prevalence of IR in female breast cancer patients using the TyG index and to identify lipid parameters associated with increased IR, thereby supporting strategies for secondary prevention.
Methods: A cross-sectional study was conducted among non-diabetic, histopathologically confirmed female breast cancer patients. Demographic data, lipid profiles, and fasting glucose levels were collected. Participants were stratified into high-risk (TyG≥8.87) and low-risk (TyG<8.87) groups based on their TyG index. Logistic regression analysis was performed to identify significant predictors of elevated TyG index.
Results: Among 122 patients, 44.3% demonstrated elevated insulin resistance. Triglycerides (TG), total cholesterol (TC), very low-density lipoprotein cholesterol (VLDL-C), and the TC/high-density lipoprotein-cholesterol (HDL-C) ratio were significantly higher in the high-risk group. Logistic regression identified TC, TC/HDL-C ratio, and low-density lipoprotein cholesterol (LDL-C) as significant predictors of elevated IR (p<0.05). The model is represented as: Logit(P)=–13.941+0.145X1+1.558X2–0.178X3, where X1, X2, and X3 correspond to TC, TC/HDL-C ratio, and LDL-C, respectively. The predictive model achieved 90.2% accuracy with an area under the receiver operating characteristic (ROC) curve (AUROC) of 0.927.
Conclusion: Monitoring lipid parameters and managing insulin resistance are crucial for enhancing breast cancer prognosis and potentially reducing progression.
Keywords
lipid profile, triglycerides, insulin resistance, breast neoplasm, logistic regression
Introduction
According to the 2020 GLOBOCAN Global Cancer Report, breast cancer has surpassed lung cancer as the most frequently diagnosed cancer in females, with an estimated 2.3 million new cases reported [1]. The situation is alarming as every 4 minutes a woman is diagnosed with breast cancer, and one woman dies from the disease every 13 minutes, largely due to late-stage diagnosis or improper prevention [2], [3]. Projections suggest that by 2030, breast cancer will become the leading cause of death among Indian women, highlighting the urgent need for effective prevention and early detection strategies [3]. Triple-negative breast cancer (TNBC) is associated with the poorest prognosis among all breast cancers [4].
It is well established that poor metabolic health increases the risk of developing breast cancer. However, recent research has shown that body mass index (BMI) alone is not a reliable indicator of metabolic health [5]. For instance, individuals with obesity (BMI>30) can still be metabolically healthy, while those who are lean (BMI<25) may experience metabolic dysfunction [5]. This highlights the importance of considering lifestyle factors – such as diet, physical activity, and stress management – in the prevention and management of metabolic health to reduce the risk of breast cancer [5].
Insulin resistance (IR) elevates insulin levels, activating pathways that promote aggressive breast cancer biology. Research on insulin analogues and anti-insulin receptor antibodies highlights the role of insulin signaling in cancer progression. IR exacerbates tumor growth by sustaining hyperinsulinemia, enhancing tumor cell proliferation and survival. Targeting IR through lifestyle changes or pharmacological interventions offers a promising strategy to improve breast cancer outcomes [6]. Additionally, IR contributes to metabolic syndrome, complicating breast cancer management. The PI3K-AKT-mTOR pathway, involved in insulin signaling, mediates resistance to endocrine therapy (ET), with high insulin receptor expression linked to poor prognosis [6].
Not all breast cancer patients exhibit insulin resistance (IR). A study by Alan et al. involving 55 patients found that 25 did not have IR and showed a better response to treatment [7]. Breast epithelial cells are surrounded by adipose tissue, and the crosstalk between these compartments is increasingly recognized as a key factor in the initiation and progression of breast cancer, alongside IR [8]. Lipid dysregulation, a common consequence of IR, leads to free fatty acid accumulation, heightening oxidative stress by increasing reactive oxygen species (ROS) production [9]. This oxidative stress damages DNA, proteins, and lipids, while the hypoxic tumor environment further exacerbates lipid accumulation in breast tissue [10]. This creates a vicious cycle that not only amplifies oxidative stress but also disrupts insulin signaling, promoting tumor growth and metastasis [10]. However, not all lipid parameter derangements predispose individuals to breast cancer. A meta-analysis of 26 prospective studies revealed conflicting data on the relationship between lipids and breast cancer [11].
The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), based on fasting glucose and insulin levels, is commonly used to evaluate IR. However, the triglyceride-glucose (TyG) index, calculated from fasting triglycerides and glucose, has emerged as a more cost-effective and reliable alternative [12], [13], [14]. This study aimed to assess the prevalence of insulin resistance in female breast cancer patients and identify the lipid parameters most significantly influencing IR to prevent further complications.
Materials and methods
This retrospective observational study was conducted at a tertiary care center in India. Newly diagnosed, histopathologically confirmed non-diabetic female breast cancer patients aged above 18 years, who provided informed consent and had complete clinical data, were included. Out of 269 patients initially enrolled, only 122 met the inclusion criteria. Patients with diabetes mellitus, those who declined consent, or those with incomplete data were excluded.
Data were collected using a pre-defined proforma covering age, BMI, smoking history, hypertension history, and family history of breast cancer. Laboratory parameters, including lipid profiles and fasting glucose, were retrieved from the hospital’s information system. Based on Panigoro et al., who identified a TyG index >8.87 as a risk threshold for breast cancer [15], participants were categorized into high- and low-risk groups accordingly.
Group I: Breast cancer patients with TyG index <8.87
Group II: Breast cancer patients with TyG index ≥8.87
The TyG index was calculated as the natural logarithm (Ln) of the product of plasma glucose and triglyceride (TG) using the formula [16]:
Difference relating to the levels of various lipid parameters and the demographic details were assessed between these two groups.
Statistical analysis plan
The data was analysed using Jamovi version 2.4.11. Normality of continuous variables was assessed with Q-Q plots and the Shapiro-Wilk test. Continuous variables were reported as mean (±SD)/median (IQR), and categorical variables as frequency and percentage. Subjects were grouped by TyG index (cutoff: 8.87). Differences between groups were analysed using independent t-test or Mann-Whitney U test for continuous variables and Chi-square test for categorical variables. A p-value ≤0.05 was considered significant. Multicollinearity among lipid parameters was assessed using the Variance Inflation Factor (VIF).
Univariate analysis was conducted to identify potential predictors, and significant variables were selected using the backward elimination method for the model. The model was developed using Python version 3.10.12, and its predictive performance was assessed to ensure goodness of fit. Odds ratios with 95% confidence intervals and standard errors were calculated for each variable. The model’s predictive value was evaluated using the receiver operating characteristic (ROC) curve, and a logistic regression formula was derived to estimate the likelihood of insulin resistance in breast cancer patients.
Various Python libraries were utilized, including NumPy for efficient numerical operations, Pandas for handling tabular data, matplotlib.pyplot for creating visualizations such as graphs, sklearn.linear_model for logistic regression modeling, sklearn.metrics for model evaluation, and statsmodels.api for advanced statistical modeling and interpretation of logistic regression results.
Results
A total of 122 breast cancer patients were recruited, with a mean age of 50.7±10.9 years (range: 26–85 years). Table 1 [Tab. 1] presents the demographic and clinical characteristics of the subjects, categorized by TyG index using a cut-off value of 8.87. There were 54 (44.3%) breast cancer patients with high IR as assessed by TyG index in our cohort. No significant differences were observed between the two groups in terms of age, BMI, history of hypertension, smoking history, low-density lipoprotein cholesterol (LDL-C) levels, high-density lipoprotein cholesterol (HDL-C) levels, LDL-C/HDL-C ratio, or family history. However, the mean or median levels of triglycerides (TG), total cholesterol (TC), very low-density lipoprotein cholesterol (VLDL-C), total cholesterol/HDL-C ratio, VLDL-C/HDL-C ratio, and triglyceride/HDL-C ratio were significantly higher in the elevated TyG index group (Table 1 [Tab. 1]).
Table 1: Demographic and clinical characteristics of subjects categorized by TyG index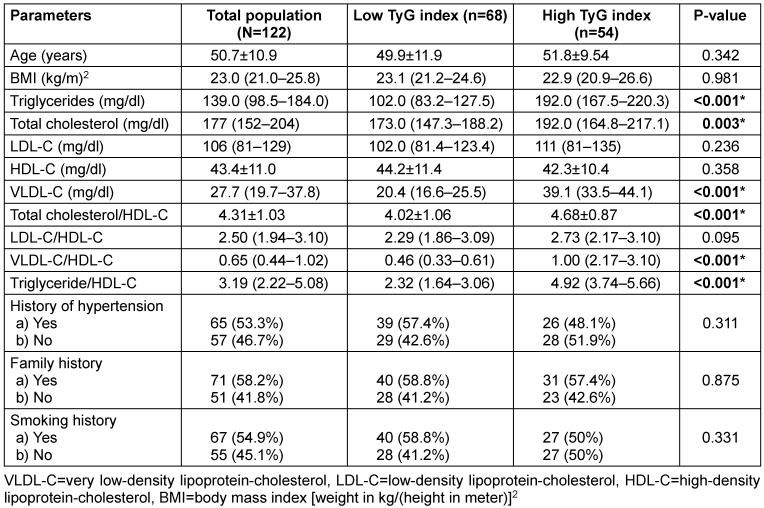
Table 2 [Tab. 2] presents the results of the univariate analysis used to identify potential parameters associated with low and elevated TyG index groups for the binary logistic regression model. Among the parameters analysed, TC, VLDL-C, TC/HDL-C ratio, VLDL-C/HDL-C ratio, and TG/HDL-C ratio showed statistical significance (P≤0.05*). Conversely, age, BMI, LDL-C, HDL-C, LDL-C/HDL-C ratio, history of hypertension, family history, and smoking history did not exhibit statistically significant differences (P>0.05).
Table 2: Univariate logistic regression analysis of parameters for predicting insulin resistance by TyG index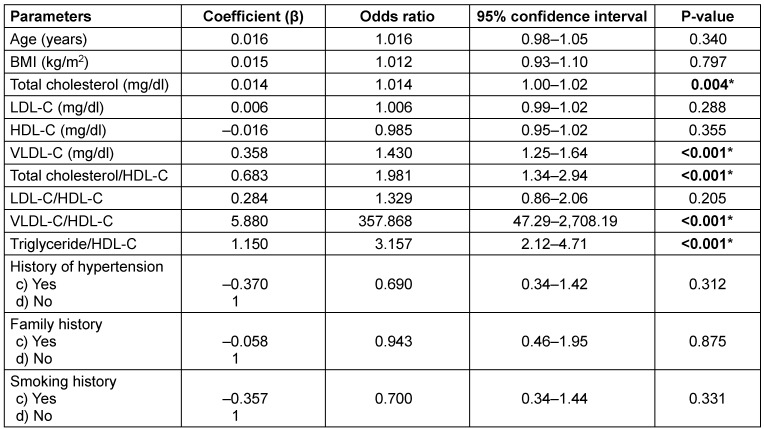
The multivariate logistic regression analysis found TC, TC/HDL-C ratio, and LDL-C as factors influencing the low and high TyG index groups in female breast cancer patients. We found no multicollinearity among these three parameters as assessed by VIF (less than 10). Thus, our results indicate that only these three are significantly associated with an IR level greater than 8.87, which is a known risk factor for the development of female breast cancer.
As shown in Table 3 [Tab. 3], the predictive model is expressed as: Logit (P)=–13.941+0.145X1+1.558X2–0.178X3, where the covariates X1, X2 and X3 represent TC, TC/HDL-C ratio, and LDL-C, respectively. So, in this logistic regression formula, on putting the values of these three parameters, we will get the logit (P) value, which will be the probability percentage of having high TyG index, indicating higher IR.
Table 3: Multivariate logistic regression analysis of parameters for predicting insulin resistance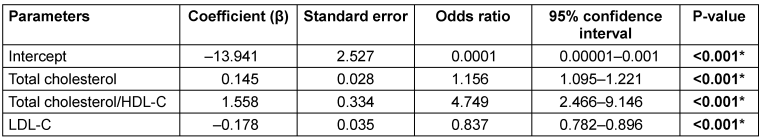
The logistic regression model demonstrated excellent predictive performance, with an area under the curve (AUC) of 0.927 (95% CI: 0.864–0.978), indicating strong discriminative ability between high and low TyG index groups. The model showed high sensitivity (85.19%) and specificity (94.12%), with a positive predictive value of 92.00% and a negative predictive value of 88.89%, resulting in an overall accuracy of 90.16%. Additionally, McFadden’s R² value of 0.39 suggests a good model fit. These findings indicate that the model reliably identifies insulin resistance using key lipid parameters. Figure 1 [Fig. 1] shows the ROC curve for the logistic regression model.
Figure 1: ROC curve of the predictive model with the area under the curve 0.927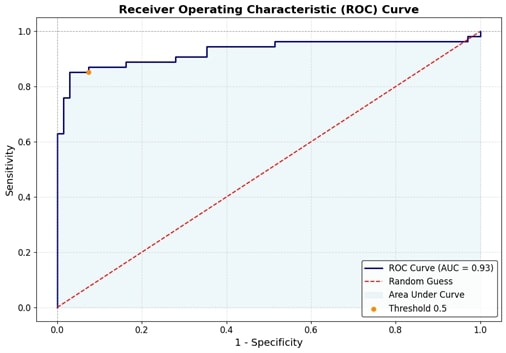
Discussion
Insulin resistance (IR) is increasingly recognized as a contributor to breast cancer progression, with the triglyceride-glucose (TyG) index serving as a reliable marker [1]. Among 122 female breast cancer patients, 44.3% exhibited high IR. The high IR group had elevated triglycerides, total cholesterol (TC), VLDL-C, and TC/HDL-C ratio. Univariate analysis identified TC, VLDL-C, and TC/HDL-C ratio as significant predictors. Multivariate regression confirmed TC, TC/HDL-C ratio, and LDL-C as independent predictors. The model demonstrated strong predictive performance (AUROC=0.927), emphasizing the clinical utility of lipid markers in IR assessment.
Chronic hyperinsulinemia promotes oncogenesis by activating cellular signaling pathways that drive growth factor-dependent cell proliferation. Insulin increases IGF-1 bioactivity by enhancing its production and suppressing IGFBP-1 and IGFBP-2 [17]. It also reduces SHBG levels, elevating free sex hormones like oestradiol and testosterone, which promote breast epithelial proliferation and inhibit apoptosis [17]. Additionally, insulin resistance (IR) induces oxidative stress via excess ROS, contributing to DNA damage and carcinogenesis [17]. Given the abundance of adipose tissue in the breast, IR, adipose-derived oestrogen, inflammatory mediators, and adipokines further amplify cancer risk, particularly in dense breast tissue, by driving oxidative stress and oncogenic signaling [8].
Dysregulated lipid metabolism has been implicated in breast cancer development, although recent meta-analyses have yielded conflicting results regarding the relationship between lipid profile parameters and breast cancer risk [14], [18]. One analysis reported a significant negative association between HDL-C levels and breast cancer risk, while no such correlation was found for TC, TG, or LDL-C [11]. Contrarily, research by Høyer et al. established a significant link between HDL-C levels and breast cancer risk, in contrast to studies by Moorman et al., Furberg et al., and Kucharska-Newton et al., which found no such association [19], [20], [21], [22]. Further investigations, including those by Vatten et al., Gaard et al., and Manjer et al., reported no significant connection between lipid measures and breast cancer prognosis [23], [24], [25]. Additionally, studies by Iso et al. and Fagherazzi et al. identified a significant correlation between TC levels and the increased risk of breast cancer complications, while other studies by Steenland et al. and Eliassen et al. found no such link [26], [27], [28], [29]. Similarly, Melvin et al. highlighted TG levels as significantly associated with higher breast cancer risk and poor prognosis, whereas Ulmer et al. found no such significant relationship [30], [31].
Our study identifies total cholesterol (TC), the TC/HDL-C ratio, and LDL-C as significant factors influencing insulin resistance (IR), as assessed by the TyG index, in female breast cancer patients. We observed that alterations in these lipid parameters are strongly associated with elevated IR, particularly when the TyG index exceeds 8.87, which is linked to a higher risk of complications and poorer prognosis, as highlighted by Panigoro et al. [15]. However, the study has limitations, including a relatively small sample size and its single-center design, which may impact the generalizability of the findings. Further research with larger, multicentre cohorts is needed to better understand the pathogenesis of complications in breast cancer patients. Early prevention and management of insulin resistance through lifestyle changes, including dietary modifications, physical activity, and pharmacological interventions, could play a crucial role in improving patient outcomes and preventing the progression of complications, ultimately leading to better prognosis in breast cancer.
Conclusion
Our study underscores the pivotal role of lipid abnormalities – specifically total cholesterol (TC), the TC/HDL-C ratio, and LDL-C – in modulating insulin resistance (IR) among women with breast cancer. We observed that these lipid imbalances are significantly linked to elevated IR, as indicated by a TyG index greater than 8.87, a well-established marker of heightened breast cancer risk. Disruptions in lipid metabolism contribute to the pathophysiology of breast cancer, potentially accelerating disease progression. These findings suggest that addressing lipid abnormalities could offer a powerful tool for early detection and preventive strategies. Regular monitoring of lipid profiles, especially for women over 30 or those perimenopausal, is critical to maintaining healthy TC, TC/HDL-C, and LDL-C levels, thereby reducing the risk of breast cancer. Moving forward, larger, multicentre studies are needed to validate these findings and explore the mechanisms by which lipid imbalances impact breast cancer outcomes. In doing so, we can improve prevention efforts and ultimately enhance patient prognosis.
Highlights
- Our study highlights the significant association between lipid abnormalities – particularly total cholesterol (TC), the TC/HDL-C ratio, and LDL-C – and elevated insulin resistance (IR) in women with breast cancer.
- Elevated IR, as indicated by a TyG index greater than 8.87, was strongly linked to an increased risk of breast cancer progression, emphasizing the role of lipid dysregulation in the disease’s pathophysiology.
- Regular monitoring of lipid profiles, particularly TC, TC/HDL-C, and LDL-C, is essential for early detection and prevention strategies, especially for women over 30 or those perimenopausal.
Data
Data for this article are available from Dryad [32] (https://doi.org/10.5061/dryad.kd51c5bjn).
Notes
Acknowledgement
We acknowledge the residents of our department for providing language help and proof reading the article.
Ethical statement
This study was approved by the Institutional Ethics Committee of All India Institute of Medical Sciences, Patna (Ref. No. AIIMS/Pat/IEC/2021/804). The requirement for informed consent was waived by the committee. All data were anonymized to maintain patient confidentiality, and the study was conducted in accordance with the ethical standards of the Declaration of Helsinki.
Authors’ contributions
Bandana Kumari contributed to the study conception, methodology and draft preparation. Bandana Kumari and Rijhul Lahariya contributed in data collection. Data analysis was performed by Rijhul Lahariya. The first draft of the manuscript was written by Bandana Kumari and Rijhul Lahariya. Both authors read and approved the final manuscript. Rijhul Lahariya and Bandana Kumari contributed equally to this work.
Authors’ ORCIDs
- Bandana Kumari: 0000-0001-5395-413X
- Rijhul Lahariya: 0009-0003-5769-4509
Competing interests
The authors declare that they have no competing interests.
References
[1] Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-49. DOI: 10.3322/caac.21660[2] Cytecare Hospitals. Statistics of Breast Cancer In India. 2019 [cited 2025 May 21]. Available from: https://cytecare.com/blog/breast-cancer/statistics-of-breast-cancer/
[3] Sofi J. Breast Cancer in India. The Times of India. 2020 Jun 30 [cited 2025 May 21]. Available from: https://timesofindia.indiatimes.com/blogs/poverty-of-ambition/breast-cancer-in-india/
[4] Wu X, Yuan F, Guo L, Gao D, Zheng W, Chen C, Zheng H, Liu J. Intraductal chemotherapy for triple-negative breast cancer: a pathway to minimally invasive clinical treatment. BMC Cancer. 2025 Feb;25(1):285. DOI: 10.1186/s12885-025-13693-0
[5] Yee LD, Mortimer JE, Natarajan R, Dietze EC, Seewaldt VL. Metabolic Health, Insulin, and Breast Cancer: Why Oncologists Should Care About Insulin. Front Endocrinol (Lausanne). 2020;11:58. DOI: 10.3389/fendo.2020.00058
[6] Javed SR, Skolariki A, Zameer MZ, Lord SR. Implications of obesity and insulin resistance for the treatment of oestrogen receptor-positive breast cancer. Br J Cancer. 2024 Dec;131(11):1724-36. DOI: 10.1038/s41416-024-02833-1
[7] Alan O, Akin Telli T, Aktas B, Koca S, Ökten IN, Hasanov R, Basoglu T, Arikan R, Demircan NC, Ercelep O, Kaya S, Ugurlu MU, Kaya H, Akgul Babacan N, Dane F, Yumuk PF. Is insulin resistance a predictor for complete response in breast cancer patients who underwent neoadjuvant treatment? World J Surg Oncol. 2020 Sep;18(1):242. DOI: 10.1186/s12957-020-02019-y
[8] Bhardwaj P, Brown KA. Obese Adipose Tissue as a Driver of Breast Cancer Growth and Development: Update and Emerging Evidence. Front Oncol. 2021;11:638918. DOI: 10.3389/fonc.2021.638918
[9] Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010 Jun;375(9733):2267-77. DOI: 10.1016/S0140-6736(10)60408-4
[10] Zipinotti Dos Santos D, de Souza JC, Pimenta TM, da Silva Martins B, Junior RSR, Butzene SMS, Tessarolo NG, Cilas PML Jr, Silva IV, Rangel LBA. The impact of lipid metabolism on breast cancer: a review about its role in tumorigenesis and immune escape. Cell Commun Signal. 2023 Jun;21(1):161. DOI: 10.1186/s12964-023-01178-1
[11] Nouri M, Mohsenpour MA, Katsiki N, Ghobadi S, Jafari A, Faghih S, Banach M, Mazidi M. Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women. J Clin Med. 2022 Aug;11(15):4503. DOI: 10.3390/jcm11154503
[12] Luo P, Cao Y, Li P, Li W, Song Z, Fu Z, Zhou H, Yi X, Zhu L, Zhu S. TyG Index Performs Better Than HOMA-IR in Chinese Type 2 Diabetes Mellitus with a BMI < 35 kg/m2: A Hyperglycemic Clamp Validated Study. Medicina (Kaunas). 2022 Jun;58(7):876. DOI: 10.3390/medicina58070876
[13] Wan H, Cao H, Ning P. Superiority of the triglyceride glucose index over the homeostasis model in predicting metabolic syndrome based on NHANES data analysis. Sci Rep. 2024 Jul;14(1):15499. DOI: 10.1038/s41598-024-66692-9
[14] Wu X, Wang S, Lin L, Jia X, Hu C, Qi H, Lin H, Zheng R, Li M, Xu Y, Xu M, Chen L, Zeng T, Hu R, Ye Z, Shi L, Su Q, Yu X, Yan L, Wang T, Zhao Z, Zheng J, Qin G, Wan Q, Chen G, Dai M, Tang X, Gao Z, Shen F, Gu X, Luo Z, Qin Y, Chen L, Hou X, Huo Y, Li Q, Wang G, Zhang Y, Liu C, Wang Y, Wu S, Yang T, Deng H, Zhao J, Mu Y, Ning G, Wang W, Bi Y, Chen Y, Lu J. Association between triglyceride glucose index and breast cancer in 142,184 Chinese adults: findings from the REACTION study. Front Endocrinol (Lausanne). 2024;15:1321622. DOI: 10.3389/fendo.2024.1321622
[15] Panigoro SS, Sutandyo N, Witjaksono F, Siregar NC, Ramli R, Hariani R, Pangarsa EA, Prajoko YW, Puruhita N, Hamdani W, Bayu D, Madjid M, Yulidar D, Fransiska JE, Widyawati R, Tripriadi ES, F W WA, Yunda DK, Pranata R. The Association Between Triglyceride-Glucose Index as a Marker of Insulin Resistance and the Risk of Breast Cancer. Front Endocrinol (Lausanne). 2021;12:745236. DOI: 10.3389/fendo.2021.745236
[16] Simental-Mendía LE, Guerrero-Romero F. The correct formula for the triglycerides and glucose index. Eur J Pediatr. 2020 Jul;179(7):1171. DOI: 10.1007/s00431-020-03644-1
[17] Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012;2012:789174. DOI: 10.1155/2012/789174
[18] Zooravar D, Radkhah H, Amiri BS, Soltani P. Association Between Triglyceride-Glucose Index and Breast Cancer: A Systematic Review and Meta-Analysis. Cancer Rep (Hoboken). 2025 Apr;8(4):e70194. DOI: 10.1002/cnr2.70194
[19] Høyer AP, Engholm G. Serum lipids and breast cancer risk: a cohort study of 5,207 Danish women. Cancer Causes Control. 1992 Sep;3(5):403-8. DOI: 10.1007/BF00051352
[20] Moorman PG, Hulka BS, Hiatt RA, Krieger N, Newman B, Vogelman JH, Orentreich N. Association between high-density lipoprotein cholesterol and breast cancer varies by menopausal status. Cancer Epidemiol Biomarkers Prev. 1998 Jun;7(6):483-8.
[21] Furberg AS, Veierød MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004 Aug;96(15):1152-60. DOI: 10.1093/jnci/djh216
[22] Kucharska-Newton AM, Rosamond WD, Mink PJ, Alberg AJ, Shahar E, Folsom AR. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol. 2008 Sep;18(9):671-7. DOI: 10.1016/j.annepidem.2008.06.006
[23] Vatten LJ, Foss OP. Total serum cholesterol and triglycerides and risk of breast cancer: a prospective study of 24,329 Norwegian women. Cancer Res. 1990 Apr;50(8):2341-6.
[24] Gaard M, Tretli S, Urdal P. Risk of breast cancer in relation to blood lipids: a prospective study of 31,209 Norwegian women. Cancer Causes Control. 1994 Nov;5(6):501-9. DOI: 10.1007/BF01831377
[25] Manjer J, Kaaks R, Riboli E, Berglund G. Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: a prospective study within the Malmö Preventive Project. Eur J Cancer Prev. 2001 Feb;10(1):33-42. DOI: 10.1097/00008469-200102000-00004
[26] Iso H, Ikeda A, Inoue M, Sato S, Tsugane S; JPHC Study Group. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer. 2009 Dec;125(11):2679-86. DOI: 10.1002/ijc.24668
[27] Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol Biomarkers Prev. 1995 Dec;4(8):807-11.
[28] Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch Intern Med. 2005 Oct;165(19):2264-71. DOI: 10.1001/archinte.165.19.2264
[29] Fagherazzi G, Fabre A, Boutron-Ruault MC, Clavel-Chapelon F. Serum cholesterol level, use of a cholesterol-lowering drug, and breast cancer: results from the prospective E3N cohort. Eur J Cancer Prev. 2010 Mar;19(2):120-5. DOI: 10.1097/CEJ.0b013e3283354918
[30] Melvin JC, Seth D, Holmberg L, Garmo H, Hammar N, Jungner I, Walldius G, Lambe M, Wigertz A, Van Hemelrijck M. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev. 2012 Aug;21(8):1381-4. DOI: 10.1158/1055-9965.EPI-12-0188
[31] Ulmer H, Borena W, Rapp K, Klenk J, Strasak A, Diem G, Concin H, Nagel G. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer. 2009 Oct;101(7):1202-6. DOI: 10.1038/sj.bjc.6605264
[32] Kumari B, Lahariya R. Data from: Evaluating lipid-driven insulin resistance via TyG index in breast cancer patients: Toward effective secondary prevention [Dataset]. Dryad; 2025. DOI: 10.5061/dryad.kd51c5bjn




