Infection induced urinary stones
1 Urology and Paediatric Urology, Regiomed-Klinikum Coburg. Medical School Regiomed, Coburg, Germany
Abstract
Stones composed of struvite, carbonate apatite and ammonium urate are regarded as induced by urinary tract infection (UTI) with bacteria producing the enzyme urease (mainly Proteus species) which splits urea into ammonium and carbonate ions resulting in supersaturation and alkalization of the urine. Struvite and apatite crystals form and grow to stones.
Compared to other mineral types infection induced stones (IIS) represent only a small percentage of urinary stones. Nevertheless they deserve special attention. Due to the high recurrence rate, the risk for loss of renal function and recurrent UTI, patients with IIS are classified as high risk stone formers.
IIS often form in patients with urinary obstruction, neurogenic bladder disorders, urinary diversion and indwelling catheters.
The mainstay in laboratory diagnosis is urinalysis, urine culture with antibiogram and stone analysis.
As IIS are regularly associated with UTI, fever at presentation is not uncommon. Then immediate imaging is required. Ultrasound of the urinary tract is the imaging modality to be used first, followed by non-contrast computerized tomography (NCCT). As in most IIS patients operative stone therapy is required, a contrast study is mandatory. Therefore, intravenous urography is also a viable option thus avoiding high radiation exposure associated with NCCT.
If there is a septic obstruction caused by the stone, immediate relief is a must. The definite stone removal should be postponed until the sepsis is resolved.
Since IIS often a large renal stones (>2 cm) or even complete or partial staghorn stone, the procedure of choice is mainly percutaneous nephrolithotomy, sometimes in combination with flexible scopes or shock wave lithotripsy. UTI must be treated prior to endourologic stone removal.
In all patients, perioperative antibiotic prophylaxis is recommended.
Specific metaphylaxis consists of removal of the stones as completely as possible, followed by antibiotic treatment (short or long term) and acidification of the urine (all these recommendations with a LE 3 and GR B).
1 Summary of recommendations
Table 1 shows the definitions for the level of evidence and the grade of recommendation.
- Due to the high recurrence rate, patients with IIS are classified as high risk stone formers (EAU guidelines urolithiasis) [1].
- The basic laboratory analysis includes urinalysis and urine culture and routine blood test like creatinine, electrolytes, uric acid, blood cell count and CRP and, if intervention is planned, blood coagulation tests (GR A). The stone analysis should be performed using a reliable method as x-ray diffraction or infrared spectroscopy. In experienced hands polarization microscopy may be used as well (LE 2; GR A).
- As they are regularly associated with UTI, fever at presentation is not uncommon. Then immediate imaging is required (LE 4; GR A).
- Ultrasound of the urinary tract is the imaging modality to be used first, followed by non-contrast computerized tomography (LE 1a; GR A). As in most IIS patients stone removal is required, a contrast study is necessary (LE 3; GR A). Therefore, intravenous urography is also a viable option (LE 4; GR C) thus avoiding high radiation exposure associated with NCCT.
- In case of septic obstruction immediate relief is mandatory (LE 1b; GR A). The definite stone therapy should be postponed until the sepsis is controlled (LE 1b; GR A).
- Since IIS often a large renal stones (>2 cm) or even complete or partial staghorn stone, the procedure of choice is mainly percutaneous nephrolithotomy, sometimes in combination with flexible ureterorenoscopy/pyeloscopy or shock wave lithotripsy (GR B).
UTI must be treated prior to endourologic stone removal (LE 1b; GR A).
In all patients, perioperative antibiotic prophylaxis is recommended (LE 1b; GR A). - Specific metaphylaxis consists of removal of the stones as completely as possible (LE 3–4; GR A), followed by antibiotic treatment (short or long term) and acidification of the urine (all these recommendations with a LE 3 and GR B). Urease inhibitors are recommended (LE 1b; GR A). However they are not available in most countries.
| Level of evidence (LE) |
Type of evidence |
| 1a | Evidence obtained from meta-analysis of randomised trials |
| 1b | Evidence obtained from at least one randomised trial |
| 2a | Evidence obtained from one well-designed controlled study without randomization |
| 2b | Evidence obtained from at least one other type of well-designed quasi-experimental study |
| 3 | Evidence obtained from well-designed non-experimental studies, such as comparative studies, correlation studies and case reports |
| 4 | Evidence obtained from expert committee reports or opinions or clinical experience of respected authorities |
| Grade of recommendation | Nature of recommendations |
| A | Based on clinical studies of good quality and consistency addressing the specific recommendations and including at least one randomised trial |
| B | Based on well-conducted clinical studies, but without randomised clinical trials |
| C | Made despite the absence of directly applicable clinical studies of good quality |
2 Introduction
The association of UTI and urolithiasis is a common phenomenon. Holmgren et al. [2] reported positive urine cultures in about 25% of their urinary stone patients. Association, however, does not necessarily imply a causal relationship. UTI can also occur as a consequence of stones.
The term “infection induced urinary stones (IIS)”, however, means that there is not only an association of stones and urinary tract infections (UTI) but a causal relationship. Already more than a hundred years ago, T.R. Brown [3] reported that some bacteria caused the formation of urinary stones consisting of calcium and magnesium phosphate. Sumner [4] isolated the bacterial enzyme urease which splits urea into ammonium and carbonate ions resulting in supersaturation and alkalization of the urine. Struvite and apatite clouds develop in which crystal growth is supported. These crystals deposit on the surface of the bacteria. Within the bacteria mainly carbonate apatite crystals form. After lysis of the bacteria, these crystals serve as new germs for stone formation (Figure 1) [5].
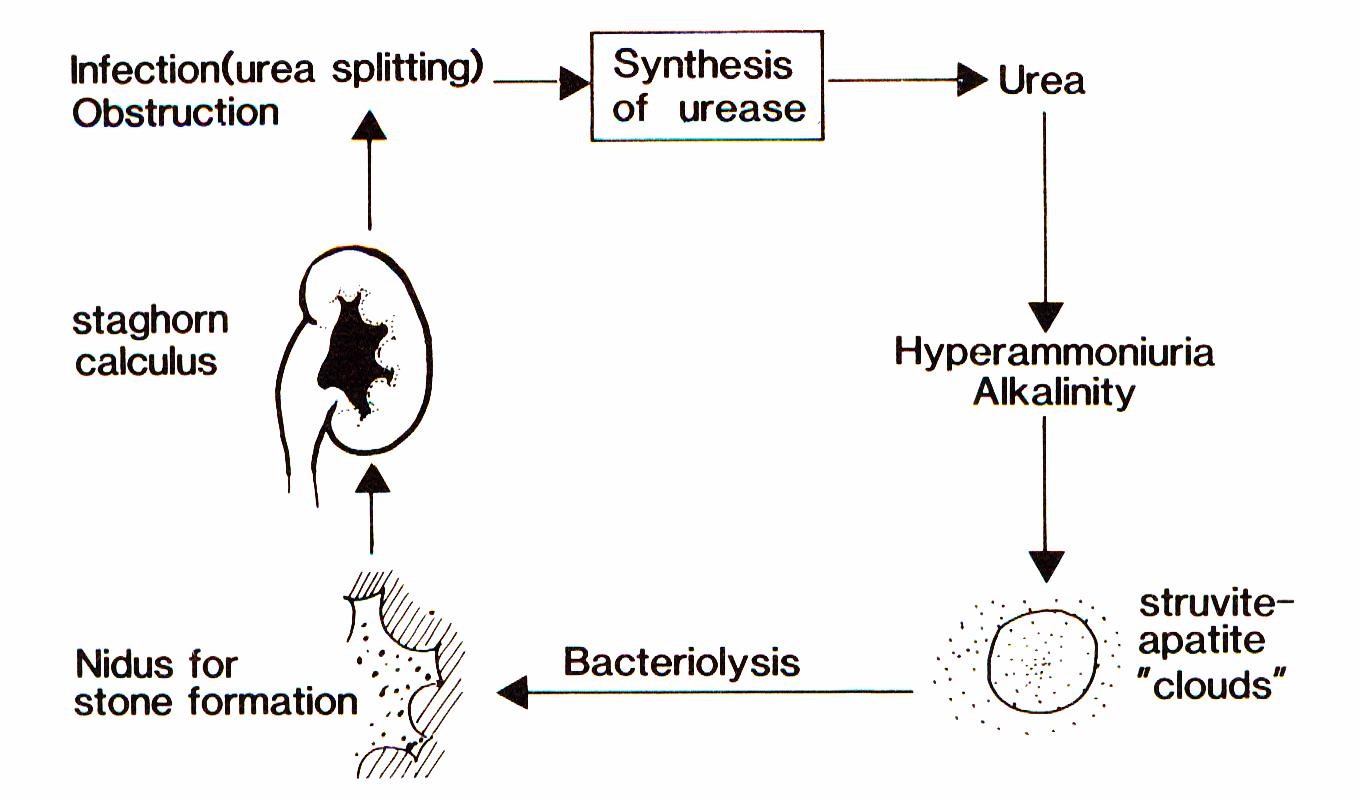
Today, three stone types are regarded as induced by UTI: struvite (magnesium ammonium phosphate), carbonate apatite (termed also carbapatite or hydroxyapaptite) and monoammonium urate [6], [7], [8].
3 Methods
A systematic literature search was performed for the last 30 years in PUBMED/MEDLINE, Cochrane etc. with the following key words: infection, urinary stones, infection induced urolithiasis, infection induced nephrolithiasis, and without limitations. A total of 231 publications were identified, which were screened by title and abstract. After exclusion of duplicates a total of 40 were included into the review (analysis), supplemented by citations of known to the author.
4 Results
Epidemiology
The current literature shows that in industrialized countries the frequency of IIS dropped considerably during the last decades. Struvite as a major component is found in 5–15 % of all stones [9], [10], [11]. Recently, in a series of almost 28,000 stone analyses Daudon et al. [12] reported a 3.5 and 12.1 percentage of struvite containing stone in males and females respectively. In the eighties of the last century, the percentages were 5.5 and 22.3 respectively.
Contrary to these figures, the percentage of IIS in developing countries is much higher ranging up to 45% [13], [14]. This reflects the fact that UTI are still more common in these countries and antibiotics are less available. Nevertheless, the percentage of IIS is also declining in these countries [15].
IIS are more common in women, infants and elderly persons [16], [17].
Pathogenesis
A UTI caused by urease producing bacteria is the mainstay of IIS [18]. This can be shown by the fact that urease production could be found in all patients with a struvite/apatite percentage of more than 80% in their stones [19].
The most important producers of this enzyme are Proteus, Morganella and Providencia species, less commonly found are urease-producing strains in Staphylococcus, Klebsiella, and Pseudomonas species [5], [19]. Furthermore, Corynebacterium urealyticum and Ureaplasma urealyticum also split urea [20], [21], [22].
The pathogenesis of struvite, which is the most frequent IIS, has been outlined already above (see Introduction).
Only in a minority of IIS carbapatite or ammonium urate are the main or solitary components.
Carbapatite cannot only form as metabolic stones (e.g. hypophosphatemia, Dent´s disease, Nephrocalcinosis) but also induced by UTI [12]. A high carbonation rate (ratio between the intensity of the ν3 band of carbonate and phosphate ions in FTIR spectroscopy) of carbapatite is suggestive for an infection induced pathway [23]. In these stones, bacterial imprints have been found by scanning electron microscopy. A carbonation rate of 15% has been proposed as a threshold [24]. Nevertheless, a high carbonation rate is not a prove for infection since in a another series it was only weakly predictive for a positive bacterial culture from the stone [25].
Ammonium urate stones are very rare today (0.2%). They form when the urine is supersaturated with both uric acid and ammonium ions resulting in alkaline urine. This may be due either to an UTI by urea-splitting bacteria or nutrition very poor in phosphate.
Clinical presentation
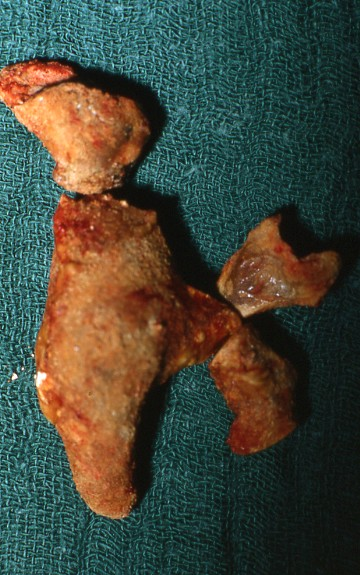
Principally, patients with IIS can present with the same symptoms (acute flank pain, renal colic, diffuse back pain) as patients with other stone types. As IIS may grow much faster than non-infected stones, usually they are larger at first presentation (Figure 2) and acute flank pain is not so common. Symptoms of UTI may accompany or precede.
IIS mainly form in conditions with a disturbed urinary flow as urinary tract obstruction on all levels (e.g. ureteropelvic junction obstruction, subvesical obstruction), neurogenic bladder dysfunction and urinary diversion. Another important risk factor is the presence of any indwelling catheter (e.g. urethral, suprapubic, ureteral, and nephrostomy). All these conditions promote UTI. In case of indwelling catheters, the formation of a biofilm additionally facilitates crystallization, crystal adherence and stone formation [26], [27].
Diagnosis
Diagnostic imaging is more or less the same as for every other stone. As they are regularly associated with UTI, fever at presentation is not uncommon. Then immediate imaging is required (LE 4; GR A).
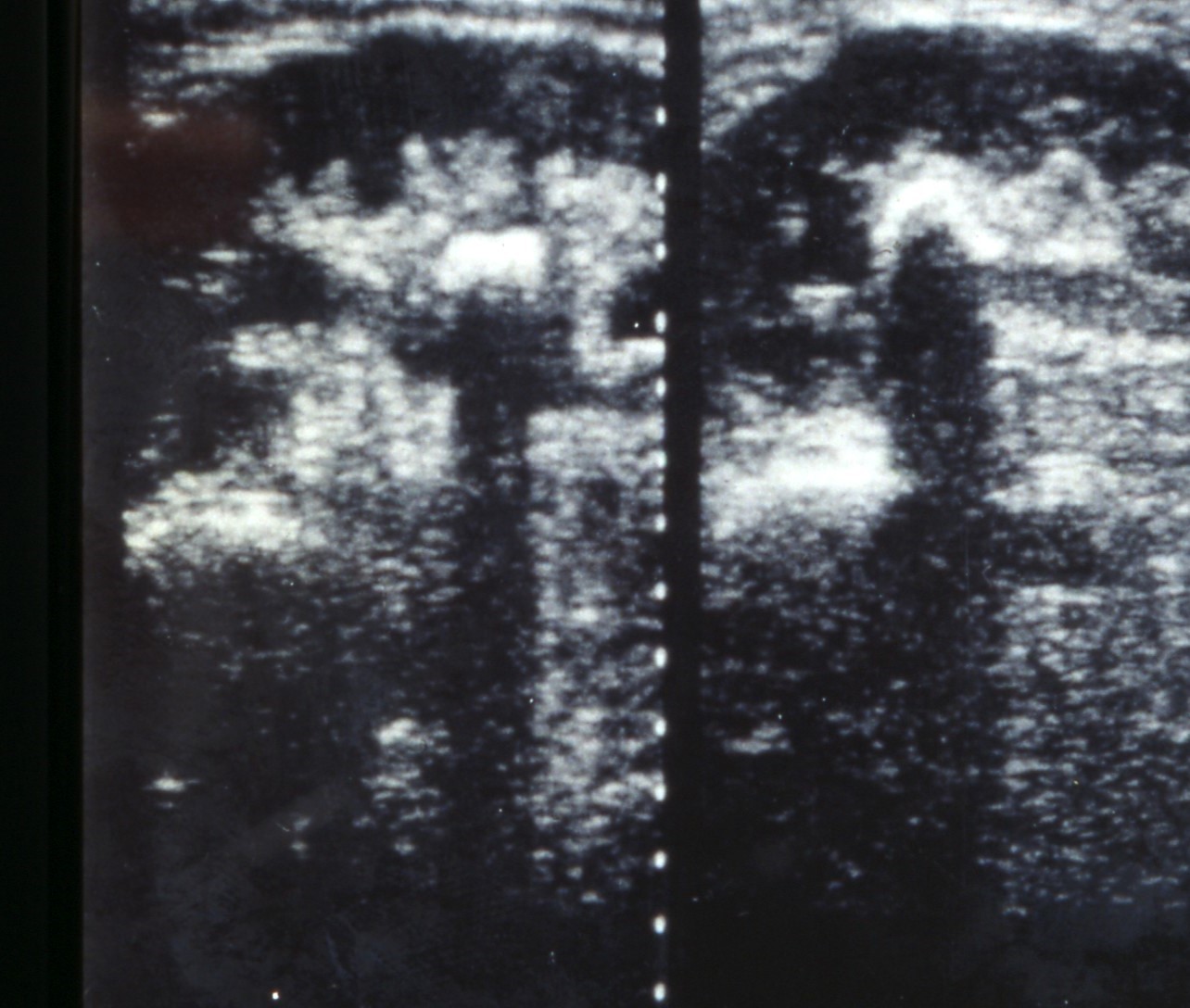
Ultrasound of the urinary tract is the imaging modality to be used first [1], [28], [29] (Figure 3). For further evaluation different radiological modalities are available. In acute flank pain, the EAU guidelines recommend to perform a non-contrast computed tomography (NCCT) as it is the method with the highest accuracy (LE 1a, GR A). [1]. However, it has to be considered that NCCT goes along with a quite high radiation dose. This dose may be lowered by using low-dose protocols. However, this is not a standardized protocol and the resolution of the NCCT is decreased considerably by lowering the radiation dose. This has to be considered especially in children. Kuhns a.o. calculated that ratio of the risk for abdominal and pelvic cancer due to a single NCCT for stones to the risk of a naturally occurring cancer over the lifetime of a child is estimated to be 2/1,000 to 3/1,000 [30].
NCCT allows for a rough estimation of the stone composition. Dependant on the mineralogical composition, IIS have a density between 400 and 1,000 Hounsfield Units [31], [32], [33], [34]. However, resolution also suffers in regard to stone composition (measurement of Hounsfield Units).
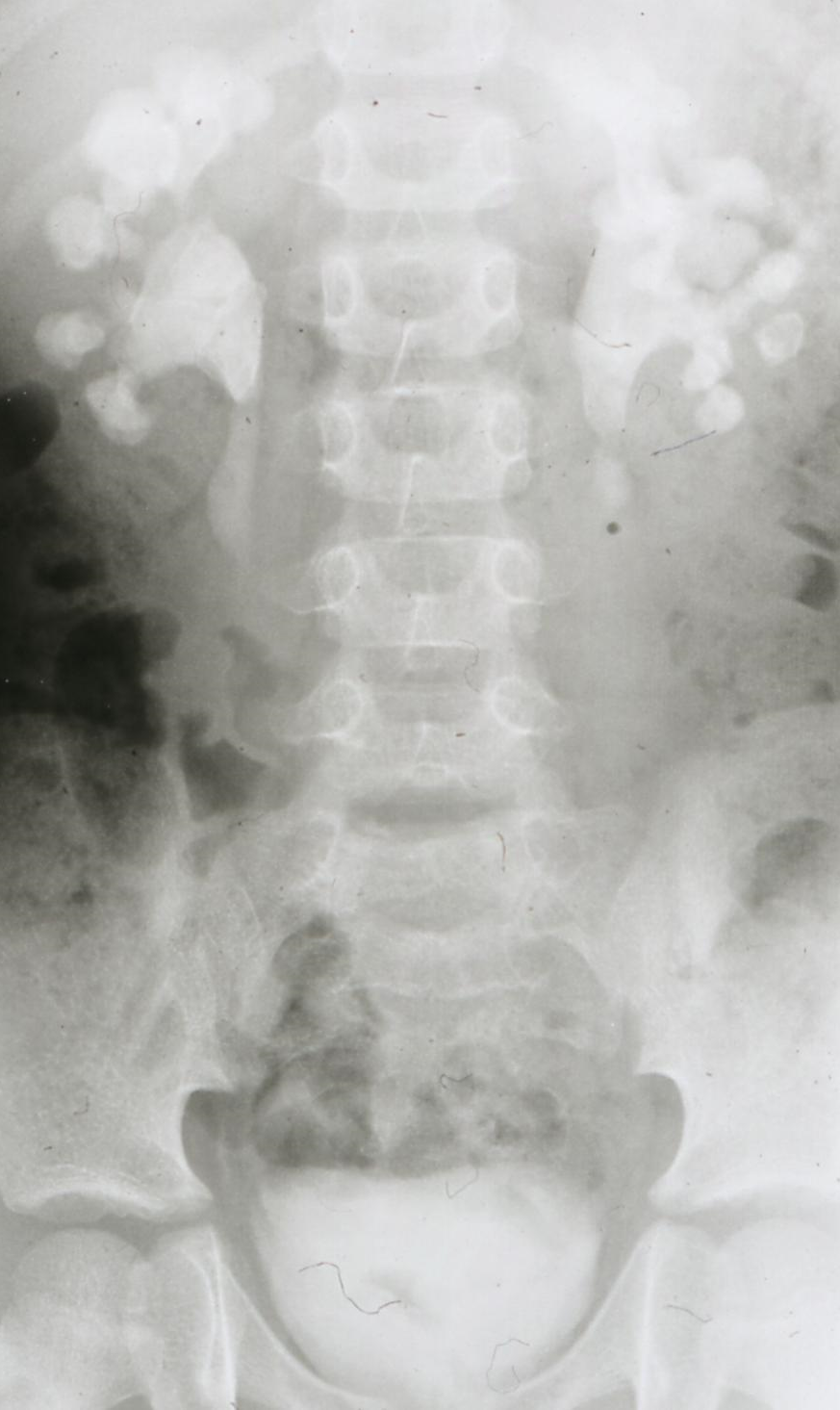
If stone removal is planned – and this is almost always the case in patients with IIS – a contrast study is necessary as the anatomy of the collecting system and renal function have to be assessed (LE 3; GR A) [1]. The EAU guidelines prefer (with a low level of evidence: 3, and low grade of recommendation: C) a contrast enhanced CT scan which means, however, a very high radiation dose of up to 10 mSv. In my opinion, an intravenous urography – which may be used also as a second choice according to the guidelines – should be preferred [29], [35] (Figure 4).
Apart from imaging, biochemical modalities are required as well. The basic laboratory analysis includes urinalysis and urine culture and routine blood test like creatinine, electrolytes, uric acid, blood cell count and CRP [1] and, if intervention is planned, blood coagulation tests (GR A). The stone analysis should be performed using a reliable method as x-ray diffraction or infrared spectroscopy. In experienced hands polarization microscopy may be used as well (LE 2; GR A) [1].
In patients with IIS, urine culture is a mainstay as these stones are induced by urease producing bacteria. A resistogram (antibiogram) is indispensable to optimize the antimicrobial therapy. A rapid urease dip-and-react test (urea-indol medium) as it is used now mainly for Helicobacter pylori diagnosis has been demonstrated to be very sensitive for urease testing in urine [5].
In patients with ammonium urate stones, a complete metabolic work-up including uric acid and phosphate measurements in blood and urine should be performed as disturbances in uric acid and phosphate metabolism may play a role for the formation of these stones.
Therapy
Principally, stone removal does not differ from other stones. The current state of the art is delineated in the EAU guidelines [1].
Since IIS often a large renal stones (>2 cm) or even complete or partial staghorn stone, the procedure of choice is mainly percutaneous nephrolithotomy, sometimes in combination with flexible ureterorenoscopy/pyeloscopy or shock wave lithotripsy (GR B). Due to the complex anatomy in these staghorn stones, stone free rates range from 50–80% [36], [37]. Therefore, in a quite large number of patients residual fragments are left behind which are the main risk factor for stone re-growth and recurrence of UTI and IIS [38].
In IIS UTI and obstruction are more common than in other stone types. Therefore, urgent relief of the obstruction (decompression) is more frequently required. For this purpose, percutaneous nephrostomy or ureteral stents may be used (LE 1b; GR A). The definitive stone therapy (e.g. percutaneous nephrolithotomy) should be postponed until the sepsis is resolved (LE 1b; GR A) [1].
In any case, in patients with (suspected) IIS, an antibiotic treatment should be started before stone removal (LE 1b; GR A). In all patients, perioperative antibiotic prophylaxis is recommended (LE 1b; GR A).
It has been shown by several authors that IIS stones may be dissolved by an irrigation therapy using Renacidin (10% hemiacridin) [39]. As it is a very time consuming and cumbersome procedure using nephrostomy and ureteral catheters and as it is not available in many countries it is no longer in use today.
Metaphylaxis (secondary prevention)
IIS stones can grow rapidly within weeks and have a high risk for recurrence [9], [40]. In case of residual fragments after stone therapy and/or persisting urinary tract infection they have a very high recurrence rate up to 70% [38], [41]. Struvite stone patients have a higher risk for loss of renal function [42]. For these reasons, patients with IIS are classified as high risk patients and need a special metaphylaxis [1].
Adequate metaphylaxis (i.e. complete removal of all fragments, consequent antibiotic therapy) can reduce the recurrence rate to 10% [43].
Therefore, the first goal is to remove the stones as completely as possible (LE 3–4; GR A). This should be followed by antibiotic treatment. The duration is still a matter of debate (short/long term). Furthermore an acidification of the urine (methionine 250–500 mg tid or ammonium chloride 1 g bid/tid) is recommended (all these recommendations with a LE 3 and GR B). Urease inhibitors like acetohydroxamic acid, the only substances studied in randomized prospective trials, have been shown to reduce the recurrence rate very effectively (LE 1b; GR A) [44], [45], [46], [47], [48]. However, due to considerable side effects, they are not available in most countries.
Recently, a pilot study using a D-mannose containing product (Cystoman®, ABI Pharmaceutical) has been used for preventing recurrences in IIS. The first results were promising, however, a definite assessment is not possible as there was no control group [49].
In ammonium urate stone formers, metabolic anomalies (e.g. hyperuricosuria) should be corrected by appropriate measures (diet, Allopurinol). For these recommendations, no randomized trials are available (LE 3–4) [1].
5 Further research
Concerning stone removal and secondary prevention (recurrence), unfortunately no real progress has been made in the last decade.
Further research should concentrate improving complete stone removal increasing the stone free rate. So far stone free rates in large IIS (50–80%) a far from being optimal. As residual fragments are the main risk factor for recurrence it is crucial too improve the stone free rates.
Also the development of less toxic urease inhibitors is an important goal as these are causal agents and the only substances with proven effectiveness in randomized studies.
6 Conclusions
Although IIS make only a quite small share of urinary stones in industrialized countries today and its incidence has decreased, they deserve closer attention as IIS stone formers are at high risk for recurrence, live threatening infections and loss of renal function.
Efforts should be made to improve the surgical results in terms of stone free rate. The current stone free rates of 50–80% in large IIS are a big problem as residual fragments are the main risk factor for re-growth of stones and recurrences.
Medical treatment and secondary prevention (metaphylaxis) should be improved as well. The most important issue is to prompt physicians to counsel and educate their patients adequately. Unfortunately, the majority of general practitioners have insufficient knowledge on stone metaphylaxis and attitude to counsel their patients [50]. Only one third of recurrent patients treated in tertiary referral centre for stone removal received adequate evaluation and metaphylaxis according to guidelines [51]. Many physicians believe that their patients would prefer to suffer from stone episodes every two years than to accept drugs for metaphylaxis. However, 88% and 92% rasp. of all stone patients would prefer to take a drug daily than to suffer from a recurrent stone episode or to undergo a new intervention for a stone [52]. Therefore, educating physicians and patients is a major goal which urgently should be pursued by our urology associations.
7 Conflict of Interest
Walter Ludwig Strohmaier is consultant of Bionorica SE, Neumarkt/Oberpfalz, Germany, and Marpinion GmbH, Oberhaching, Germany.
References
[1] Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU Guidelines on Diagnosis and Conservative Management of Urolithiasis. Eur Urol. 2016 Mar;69(3):468-74. DOI: 10.1016/j.eururo.2015.07.040[2] Holmgren K, Danielson BG, Fellström B, Ljunghall S, Niklasson F, Wikström B. The relation between urinary tract infections and stone composition in renal stone formers. Scand J Urol Nephrol. 1989;23(2):131-6.
[3] Brown TR. On the relation between the variety of micro-organisms and the composition of stone in calculous pyelonephritis. J Am Med Assoc. 1901 May 18;XXXVI(20):1395-7. DOI: 10.1001/jama.1901.52470200035001k
[4] Sumner JB. The isolation and crystallization of the enzyme urease. J Biol Chem. 1926;69:435-441.
[5] Strohmaier WL, Bichler KH, Naber K. Valency of the UREA (R) dip-and-react test for detecting urea-splitting bacteria. In: Martelli A, Buli P, Marchesini B. International Meeting on Inhibitors of Crystallization in Renal Lithiasis and Their Clinical Application: Bologna September 7-8-9th, 1987. Rome: Acta Medica; 1988. p. 265-9.
[6] Bichler KH, Eipper E, Naber K. Infektinduzierte Harnsteine [Infection-induced urinary stones]. Urologe A. 2003 Jan;42(1):47-55. DOI: 10.1007/s00120-002-0272-5
[7] Meissner A, Mamoulakis C, Laube N. Harnwegsinfektionen und Urolithiasis [Urinary tract infections and Urolithiasis]. Urologe A. 2010 May;49(5):623-8. DOI: 10.1007/s00120-010-2257-0
[8] Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int. 2007;79 Suppl 1:32-6. DOI: 10.1159/000104439
[9] Cohen TD, Preminger GM. Struvite calculi. Semin Nephrol. 1996 Sep;16(5):425-34.
[10] Leusmann DB, Blaschke R, Schmandt W. Results of 5,035 stone analyses: a contribution to epidemiology of urinary stone disease. Scand J Urol Nephrol. 1990;24(3):205-10.
[11] Leusmann DB, Niggemann H, Roth S, von Ahlen H. Recurrence rates and severity of urinary calculi. Scand J Urol Nephrol. 1995 Sep;29(3):279-83.
[12] Daudon M, Bouzidi H, Bazin D. Composition and morphology of phosphate stones and their relation with etiology. Urol Res. 2010 Dec;38(6):459-67. DOI: 10.1007/s00240-010-0320-3
[13] Daudon M, Bounxouei B, Santa Cruz F, Leite da Silva S, Diouf B, Angwafoo FF 3rd, Talati J, Desrez G. Composition des calculs observés aujourd'hui dans les pays non industrialisés [Composition of renal stones currently observed in non-industrialized countries]. Prog Urol. 2004 Dec;14(6):1151-61.
[14] Strohmaier WL. Course of renal stone disease - an epidemiological view. In: Gohel MDI, Au DWT, editors. Kidney stones : inside & out : proceedings of the 10th International Symposium on Urolithiasis, held on May 25-28, 2004, in Hong Kong, China. Hong Kong: Reprographic Unit, The Hong Kong Polytechnic University; 2004. p. 377-385.
[15] Djelloul Z, Djelloul A, Bedjaoui A, Kaid-Omar Z, Attar A, Daudon M, Addou A. Lithiase urinaire dans l'Ouest algérien: étude de la composition de 1354 calculs urinaires en relation avec leur localisation anatomique, l'âge et le sexe des patients [Urinary stones in Western Algeria: study of the composition of 1,354 urinary stones in relation to their anatomical site and the age and gender of the patients]. Prog Urol. 2006 Jun;16(3):328-35.
[16] Borghi L, Ferretti PP, Elia GF, Amato F, Melloni E, Trapassi MR, Novarini A. Epidemiological study of urinary tract stones in a northern Italian city. Br J Urol. 1990 Mar;65(3):231-5.
[17] Clark JY. Renal calculi in army aviators. Aviat Space Environ Med. 1990 Aug;61(8):744-7.
[18] Bichler K-H, Eipper E, Naber K, Braun V, Zimmermann R, Lahme S. Urinary infection stones. Int J Antimicrob Agents. 2002 Jun;19(6):488–98.DOI: 10.1016/S0924-8579(02)00088-2
[19] Balk N, Strohmaier WL, Schmid M, Bichler KH. Bacteriologic and metabolic findings in patients with infected urinary stones [Bakteriologische und metabolische Befund bei Patienten mit Urolithiasis]. Urol Res. 1990;18(1):66. DOI: 10.1007/BF00294588
[20] Hedelin H, Brorson JE, Grenabo L, Pettersson S. Ureaplasma urealyticum and upper urinary tract stones. Br J Urol. 1984 Jun;56(3):244-9.
[21] Kaya S, Poyraz O, Gökçe G, Kiliçarslan H, Kaya K, Ayan S. Role of genital mycoplasmata and other bacteria in urolithiasis. Scand J Infect Dis. 2003;35(5):315-7. DOI: 10.1080/00365540310004018
[22] Rose GA, Rosenbaum TP. Recurrent infection stones with apparently negative cultures. The case for blind antibacterial treatment. Br J Urol. 1992 Mar;69(3):234-9.
[23] Carpentier X, Daudon M, Traxer O, Jungers P, Mazouyes A, Matzen G, Véron E, Bazin D. Relationships between carbonation rate of carbapatite and morphologic characteristics of calcium phosphate stones and etiology. Urology. 2009 May;73(5):968-75. DOI: 10.1016/j.urology.2008.12.049
[24] Maurice-Estepa L, Levillain P, Lacour B, Daudon M. Crystalline phase differentiation in urinary calcium phosphate and magnesium phosphate calculi. Scand J Urol Nephrol. 1999 Oct;33(5):299-305.
[25] Englert KM, McAteer JA, Lingeman JE, Williams JC Jr. High carbonate level of apatite in kidney stones implies infection, but is it predictive? Urolithiasis. 2013 Oct;41(5):389-94. DOI: 10.1007/s00240-013-0591-6
[26] Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008 Jan;21(1):26-59. DOI: 10.1128/CMR.00019-07
[27] Paick J-S, Hong SK, Park M-S, Kim SW. Management of postoperatively detected iatrogenic lower ureteral injury: Should ureteroureterostomy really be abandoned? Urology. 2006 Feb;67(2):237–41. DOI: 10.1016/j.urology.2005.08.041
[28] Strohmaier W. Diagnostic imaging in pediatric urolithiasis. J Pediatr Biochem. 2016 Aug 3;4(2):081–8. DOI: 10.3233/JPB-140110
[29] Strohmaier WL, Bartunek R. [Diagnostic imaging--the end of intravenous urography?]. Urologe A. 2008 May;47(5):556, 558-62. DOI: 10.1007/s00120-008-1709-2
[30] Kuhns LR, Oliver WJ, Christodoulou E, Goodsitt MM. The predicted increased cancer risk associated with a single computed tomography examination for calculus detection in pediatric patients compared with the natural cancer incidence. Pediatr Emerg Care. 2011 Apr;27(4):345-50. DOI: 10.1097/PEC.0b013e3182132016
[31] Bellin MF, Renard-Penna R, Conort P, Bissery A, Meric JB, Daudon M, Mallet A, Richard F, Grenier P. Helical CT evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of CT-attenuation values and density. Eur Radiol. 2004 Nov;14(11):2134-40. DOI: 10.1007/s00330-004-2365-6
[32] Burgos FJ, Sánchez J, Avila S, Saez JC, Escudero Barrilero A. Utilidad de la tomografía axial computarizada (CT) en el establecimiento de la composición litiásica [The usefulness of computerized axial tomography (CT) in establishing the composition of calculi]. Arch Esp Urol. 1993 Jun;46(5):383-91.
[33] Newhouse JH, Prien EL, Amis ES Jr, Dretler SP, Pfister RC. Computed tomographic analysis of urinary calculi. AJR Am J Roentgenol. 1984 Mar;142(3):545-8. DOI: 10.2214/ajr.142.3.545
[34] Sheir KZ, Mansour O, Madbouly K, Elsobky E, Abdel-Khalek M. Determination of the chemical composition of urinary calculi by noncontrast spiral computerized tomography. Urol Res. 2005 May;33(2):99-104. DOI: 10.1007/s00240-004-0454-2
[35] Strohmaier WL. Imaging in pediatric urolithiasis-what's the best choice? Transl Pediatr. 2015 Jan;4(1):36-40. DOI: 10.3978/j.issn.2224-4336.2015.01.01
[36] el-Nahas AR, Eraky I, Shokeir AA, Shoma AM, el-Assmy AM, el-Tabey NA, Soliman S, Elshal AM, el-Kappany HA, el-Kenawy MR. Factors affecting stone-free rate and complications of percutaneous nephrolithotomy for treatment of staghorn stone. Urology. 2012 Jun;79(6):1236-41. DOI: 10.1016/j.urology.2012.01.026
[37] Shahrour K, Tomaszewski J, Ortiz T, Scott E, Sternberg KM, Jackman SV, Averch TD. Predictors of immediate postoperative outcome of single-tract percutaneous nephrolithotomy. Urology. 2012 Jul;80(1):19-25. DOI: 10.1016/j.urology.2011.12.065
[38] Beck EM, Riehle RA Jr. The Fate of Residual Fragments after Extracorporeal shock wave Lithotripsy Monotherapy of Infection Stones. J Urol. 1991 Jan;145(1):6–9.DOI: 10.1016/S0022-5347(17)38230-7
[39] Gonzalez RD, Whiting BM, Canales BK. The history of kidney stone dissolution therapy: 50 years of optimism and frustration with renacidin. J Endourol. 2012 Feb;26(2):110-8. DOI: 10.1089/end.2011.0380
[40] Griffith DP, Osborne CA. Infection (urease) stones. Miner Electrolyte Metab. 1987;13(4):278-85.
[41] Martínez-Piñeiro JA, de Iriarte EG, Armero AH. The problem of recurrences and infection after surgical removal of staghorn calculi. Eur Urol. 1982;8(2):94-101.
[42] Gambaro G, Favaro S, D'Angelo A. Risk for renal failure in nephrolithiasis. Am J Kidney Dis. 2001 Feb;37(2):233-43.
[43] Jarrar K, Boedeker RH, Weidner W. Struvite stones: long term follow up under metaphylaxis. Ann Urol (Paris). 1996;30(3):112-7.
[44] Griffith DP, Gibson JR, Clinton CW, Musher DM. Acetohydroxamic Acid: Clinical Studies of a Urease Inhibitor in Patients With Staghorn Renal Calculi. J Urol. 1978 Jan;119(1):9–15. DOI: 10.1016/S0022-5347(17)57366-8
[45] Griffith DP, Gleeson MJ, Lee H, Longuet R, Deman E, Earle N. Randomized, double-blind trial of Lithostat (acetohydroxamic acid) in the palliative treatment of infection-induced urinary calculi. Eur Urol. 1991;20(3):243-7.
[46] Griffith DP, Khonsari F, Skurnick JH, James KE. A Randomized Trial of Acetohydroxamic Acid for the Treatment and Prevention of Infection-Induced Urinary Stones in Spinal Cord Injury Patients. J Urol. 1988 Aug;140(2):318–24. DOI: 10.1016/S0022-5347(17)41592-8
[47] Griffith DP, Musher DM. Prevention of infected urinary stones by urease inhibition. Invest Urol. 1973 Nov;11(3):228-33.
[48] Griffith DP, Musher DM. Acetohydroxamic acid. Urology. 1975 Mar;5(3):299–302. DOI: 10.1016/0090-4295(75)90142-9
[49] Proietti S, Giannantoni A, Luciani LG, Sortino G, Graziotti P, Giusti G. Cystoman® and calculi: a good alternative to standard therapies in preventing stone recurrence. Urolithiasis. 2014 Aug;42(4):285-90. DOI: 10.1007/s00240-014-0675-y
[50] Bos D, Abara E, Parmar MS. Knowledge, attitudes, and practice patterns among healthcare providers in the prevention of recurrent kidney stones in Northern Ontario. Can Urol Assoc J. 2014 Nov;8(11-12):E795-804. DOI: 10.5489/cuaj.1455
[51] Krepinsky J, Ingram AJ, Churchill DN. Metabolic investigation of recurrent nephrolithiasis: compliance with recommendations. Urology. 2000 Dec;56(6):915–20. DOI: 10.1016/S0090-4295(00)00822-0
[52] Bensalah K, Tuncel A, Raman JD, Bagrodia A, Pearle M, Lotan Y. How physician and patient perceptions differ regarding medical management of stone disease. J Urol. 2009 Sep;182(3):998-1004. DOI: 10.1016/j.juro.2009.05.025




