Building a digital platform for collaborative second opinions in rare disease: Integrating AI and healthcare networks for improved care
Vinícius Lima 1,2Filipe Bernardi 1,2
Victor Ferraz 1
Domingos Alves 1
1 Ribeirão Preto Medical School, University of São Paulo, Brazil
2 RISE-Health, Faculty of Medicine, University of Porto, Portugal
Abstract
The RD2O platform is a digital solution designed to support second opinions in rare disease care, particularly in healthcare systems marked by regional disparities, such as Brazil’s. Developed through action research and grounded in a sociotechnical approach, RD2O connects general practitioners, specialists, and patient associations via a secure web portal. The platform allows the submission of clinical and non-clinical queries, which are supported by generative AI models and validated by human experts. Preliminary evaluations demonstrate that RD2O streamlines complex case discussions, enhances the accuracy of second opinions, and contributes to a growing, publicly accessible educational database. Its modular architecture integrates clinical guidelines, privacy protocols, and telehealth tools, making it scalable and adaptable to other national contexts. RD2O ensures ethical, user-centered, and evidence-based digital health transformation by embedding evaluation mechanisms at every stage of development. This platform exemplifies how collaborative, AI-assisted systems can strengthen RD care, inform public policy, and generate validated knowledge through a secure, auditable process.
Keywords
rare diseases, second opinion, digital health, generative AI
Introduction
Rare diseases (RDs) affect a small fraction of the population individually but collectively represent a significant global public health concern. With over 7,000 recognized rare diseases, most of them complex and chronically debilitating, patients often endure lengthy diagnostic odysseys, inappropriate treatments, and considerable emotional and financial burdens [1]. In countries like Brazil, the situation is further complicated by stark regional disparities in healthcare access and the distribution of medical specialists [2].
Second opinions from specialized professionals are often critical to improving diagnostic precision, ensuring appropriate therapeutic approaches, and reducing the risk of unnecessary or harmful interventions [3].
This paper presents the Rare Disease Second Opinion (RD2O) platform, an evidence-based digital initiative developed in Brazil to support the delivery of second opinions for patients with rare diseases. Inspired by international models such as the Clinical Patient Management System (CPMS) [4], and EURACAN [5], RD2O brings an inclusive approach anchored in Brazil’s legal frameworks.
Methods
The RD2O platform was conceived using the action research methodology [6], which allows for continuous co-construction of knowledge and solution refinement in real-world contexts. This iterative and participatory process involved diverse stakeholders, including physicians, healthcare administrators, IT professionals, and researchers. The platform is under development in the Clinics Hospital of Ribeirão Preto, supported by the Brazilian National Rare Disease Network (RARAS) and the National Institute of Rare Diseases (INCT).
Stakeholder engagement was a central component of the design process. Multiple interactions were conducted to refine features and identify potential barriers to adoption. Healthcare professionals contributed insights into practical challenges, including the standardization of medical records and the need for context-specific clinical guidelines. Platform development was structured into the following phases:
- Theoretical and empirical groundwork based on national and international experiences, including the Formative Second Opinion from the Brazilian Telehealth Program [7];
- Mapping user needs, legal requirements, and system limitations;
- Solution modeling using Business Process Model and Notation (BPMN) to define clinical workflows, content approval hierarchies, and user roles; and
- System database modeling, designing of interactive prototypes with FIGMA, and iterative testing.
The technical architecture combines Python with MySQL and MongoDB to handle relational and document-based data. Generative AI models (e.g., ChatGPT, Gemini, and Llama) will automate drafts of responses and streamline query processing. Human experts review all AI-generated outputs to ensure clinical rigor.
Preliminary results
Preliminary results from the first phase demonstrate the platform’s potential to streamline the process of obtaining second opinions for cases involving rare diseases. The RD2O web portal is proposed as a centralized access point. Through this interface, users will be able to:
- submit detailed clinical cases for expert group discussion;
- submit non-clinical or administrative queries (e.g., disease classification, guideline development, or policy alignment); and
- access a public knowledge database of previously validated second opinions and clinical insights.
Figure 1 [Fig. 1] presents the platform overview.
Figure 1: RD2O platform overview. Outputs include structured case discussions, technical Q&A, and anonymized expert reports, all searchable via a public educational portal.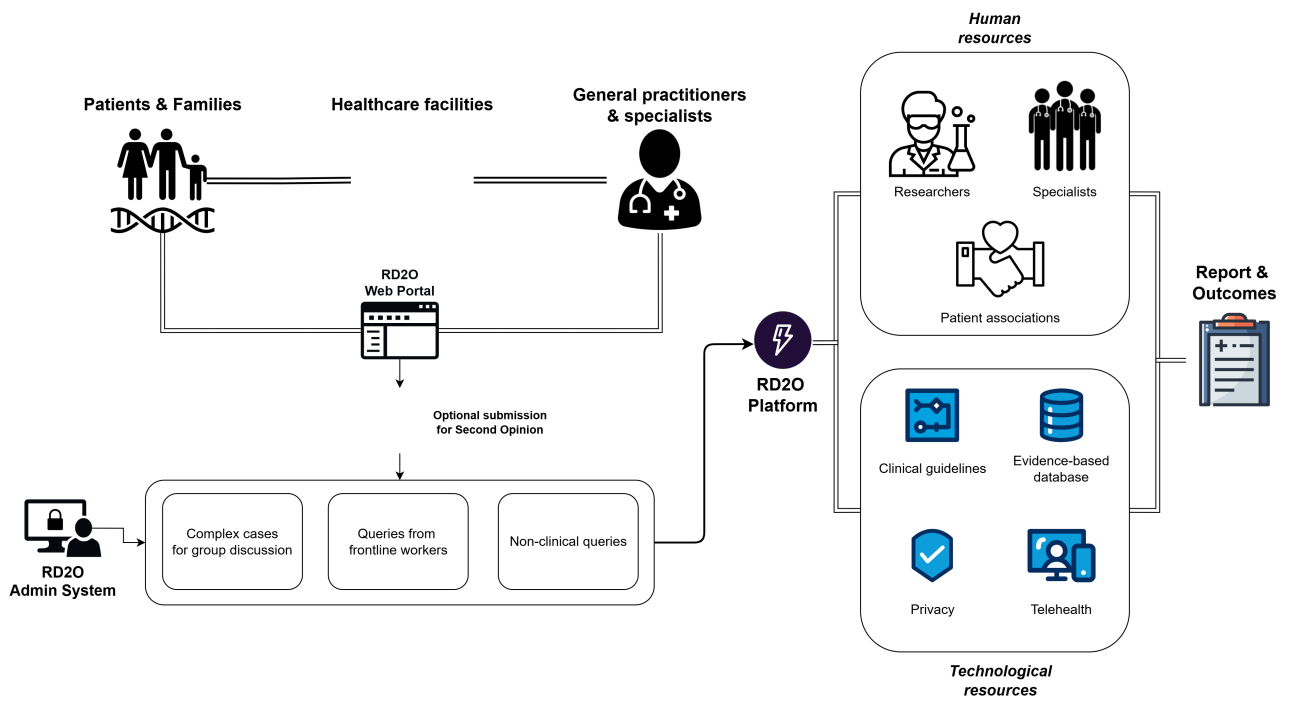
Each query submitted will be processed through the RD2O Admin System, where it will be classified and routed to the appropriate reviewers. AI models will support this process by generating draft responses or literature summaries, thereby reducing turnaround times. Case discussions and documentation will contribute to a continuously growing educational database, supporting the dissemination of knowledge. An AI audit trail is embedded to assess model reliability and track concordance between human expert responses and AI-generated content. Metrics for validation include time-to-response, clinical relevance scoring, and usability feedback from pilot users.
Final considerations
The development of the RD2O platform represents a pivotal step in improving the management and care of rare diseases in Brazil. The platform supports healthcare professionals with practical, validated insights and fosters a culture of continuous learning through its educational database. The modular architecture may support interoperability with national systems. Sustainability is addressed through key partnerships (RARAS, INCT) and engagement in public policy.
Notes
Acknowledgments
The São Paulo Research Foundation (FAPESP) supports this project (grant no. 2023/10203-8 and 2024/22679-0).
Competing interests
The authors declare that they have no competing interests.
References
[1] Marwaha S, Knowles JW, Ashley EA. A guide for the diagnosis of rare and undiagnosed disease: beyond the exome. Genome Med. 2022 Feb 28;14(1):23. DOI: 10.1186/s13073-022-01026-w[2] Machado CV, Silva GAE. Political struggles for a universal health system in Brazil: successes and limits in the reduction of inequalities. Global Health. 2019 Nov 28;15(Suppl 1):77. DOI: 10.1186/s12992-019-0523-5
[3] Hayeems RZ, Michaels-Igbokwe C, Venkataramanan V, Hartley T, Acker M, Gillespie M, Ungar WJ, Mendoza-Londona R, Bernier FP, Boycott KM, Marshall DA. The complexity of diagnosing rare disease: An organizing framework for outcomes research and health economics based on real-world evidence. Genet Med. 2022 Mar;24(3):694-702. DOI: 10.1016/j.gim.2021.11.005
[4] Mönig I, Steenvoorden D, de Graaf JP, Ahmed SF, Taruscio D, Beun JG, Johannsen TH, Juul A, Hiort O, Pereira AM. CPMS-improving patient care in Europe via virtual case discussions. Endocrine. 2021 Mar;71(3):549-54. DOI: 10.1007/s12020-021-02628-x
[5] Blay JY, Casali P, Ray-Coquard I, Seckl MJ, Gietema J, de Herder WW, Caplin M, Klümpen HJ, Glehen O, Wyrwicz L, Peeters R, Licitra L, Girard N, Piperno-Neumann S, Kapiteijn E, Idbaih A, Franceschi E, Trama A, Frezza AM, Hohenberger P, Hindi N, Martin-Broto J, Schell J, Rogasik M, Lejeune S, Oliver K, de Lorenzo F, Weinman A. Management of patients with rare adult solid cancers: objectives and evaluation of European reference networks (ERN) EURACAN. Lancet Reg Health Eur. 2024 Feb 16;39:100861. DOI: 10.1016/j.lanepe.2024.100861
[6] Greenwood DJ, Levin M. Introduction to Action Research. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2006.
[7] Haddad AE, Skelton-Macedo MC, Abdala V, Bavaresco C, Mengehel D, Abdala CG, Harzheim E. Formative second opinion: qualifying health professionals for the unified health system through the Brazilian Telehealth Program. Telemed J E Health. 2015 Feb;21(2):138-42. DOI: 10.1089/tmj.2014.0001




