A comprehensive review on the epidemiology of arboviruses in the Eastern Mediterranean Region (EMRO): insights from the WHO’s Regional Office
Iman Owliaee 1Mehran Khaledian 2
Ali Shojaeian 3
Farid Azizi Jalilian 1
1 Department of Medical Virology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
2 Department of Medical Entomology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
3 Research Center for Molecular Medicine, Institute of Cancer, Avicenna Health Research Institute, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Arboviruses (arthropod-borne viruses) pose an ongoing public health threat in the Eastern Mediterranean Region (EMRO) of the World Health Organization. This review summarizes the epidemiology of major arboviruses – including Crimean-Congo hemorrhagic fever virus (CCHFV), chikungunya virus (CHIKV), dengue virus (DENV), Rift Valley fever virus (RVFV), West Nile virus (WNV), yellow fever virus (YFV), and Zika virus (ZIKV) in EMRO countries based on data from 2014–2023. Reported prevalence rates varied considerably between studies and countries, indicating localized transmission intensity. Overall, serological evidence confirms endemic circulation of CCHFV, CHIKV, DENV, and WNV in parts of the region. Large DENV outbreaks highlight it as a key concern. More systematic surveillance and standardized diagnostics are needed to characterize arbovirus epidemiology across the region and inform control strategies.
Keywords
arboviruses, arthropod-borne viruses, Crimean-Congo hemorrhagic fever virus, chikungunya virus, dengue virus, Rift Valley fever virus, West Nile virus, yellow fever virus, Zika virus, epidemiology, EMRO, Eastern Mediterranean countries
Introduction
Virology
Arthropod-borne viruses, or arboviruses, constitute an ongoing and formidable risk to human and veterinary health [1], [2]. This diverse group of pathogens, which includes over 500 distinct viruses, is disseminated primarily through hematophagous arthropods, including mosquitoes, ticks, midges, and other blood-feeding insects, and through virus-infected vertebrate hosts [3]. Arboviruses are a major public health threat in the Eastern Mediterranean Region (EMRO) of the World Health Organization (WHO), which comprises 21 countries from Morocco to Pakistan [4], [5]. However, alternative transmission pathways within vertebrate populations have also been documented independently of arthropod vectors. These alternative pathways include mother-to-child transmission, nosocomial infections, subsequent transmission via blood transfusions or organ transplantation, and sexual transmission. Often viewed as incidental or secondary hosts in the viral life cycle, humans find themselves embroiled in the complex network of arboviral infections [6]. The past few decades have seen a rise in infectious diseases caused by arboviruses, leading to considerable loss of human and livestock lives and significant economic impacts [7]. Among this group of pathogens, several are implicated in severe diseases with global impact, and many have the potential to spark emerging infectious outbreaks. Examples of these virulent agents include dengue virus (DENV), chikungunya virus, West Nile virus (WNV), Japanese encephalitis virus (JEV), tick-borne encephalitis virus (TBEV), yellow fever virus (YFV), Rift Valley fever virus (RVFV), Sindbis virus (SINV), Crimean-Congo Hemorrhagic Fever virus (CCHF), and Zika virus (ZIKV) [8], [9].
The term “arbovirus” is a broad range of RNA viruses capable of infecting humans. This includes alphaviruses from the Togaviridae family, flaviviruses from the Flaviviridae family, bunyaviruses, nairoviruses, phleboviruses from the Bunyaviridae family, orbiviruses from the Reoviridae family, and vesiculoviruses [10]. Co-infections, especially those involving ZIKV and CHIKV, are widely reported and are thought to increase vector transmission and intensify ZIKV pathogenicity [10].
Diagnostics
Differential diagnoses of such arboviral infections pose a complex challenge due to their often indistinguishable early-stage clinical symptoms. While molecular diagnostics are highly specific, they exhibit optimal sensitivity only during the acute phase of infection and can present logistical challenges in resource-limited endemic regions [7]. Several methods have been developed to detect the presence of these pathogens in clinical samples, including enzyme-linked immunosorbent assays (ELISAs), lateral flow assays (LFAs), and reverse transcriptase-polymerase chain reaction (RT-PCR) [11].
Epidemiology
To date, 71 mosquito species have been recorded in Iran [12], [13]. Among these species, those belonging to the Culex and Aedes genera have emerged as the primary vectors of arboviruses [14]. In recent years, a surge in population movements, including refugee flows, has occurred, and there has been increased cross-border trade in goods and animals across regions like the EMRO. This region includes countries like Pakistan, which have experienced a significant rise in the burden of arboviral diseases [15], [16]. Understanding arboviruses is paramount in this context, given their profound impact on public health within the EMRO region. Arboviral diseases pose a substantial threat to the population in numerous EMRO countries. Through rigorous study, health authorities can gain insights into the epidemiology, transmission dynamics, and potential outbreak patterns of these viruses, thereby facilitating the development of effective prevention strategies and timely interventions to curtail the spread of arboviral disease [17]. Furthermore, examining arboviruses in the EMRO region is pivotal for developing diagnostic tools and treatment modalities. By elucidating specific viral strains and their genetic attributes, researchers can tailor targeted therapeutic approaches to combat these diseases with greater efficacy [18]. Additionally, studying arboviruses in this region enables the identification of potential reservoirs and vectors, a crucial step in implementing effective vector control measures and reducing the risk of future outbreaks [19]. Moreover, by sharing this knowledge with neighboring regions and international health organizations, EMRO can contribute significantly to global efforts to prevent the proliferation of arboviral diseases.
This article provides an overview of the epidemiology of major arboviral diseases in EMRO countries, including their distribution, burden, outbreaks, and arthropod vectors. Understanding the epidemiological patterns and risk factors for arboviral transmission is critical for surveillance, preparedness, and response to mitigate the public health impact of these diseases in the region.
Methods
A literature search was conducted in January 2024 to identify relevant studies on the epidemiology of major arboviruses in EMRO countries published during 2014–2023. To discover related studies, we conducted a comprehensive search of international databases such as PubMed, Scopus, Science Direct, Cochrane, Embase, Web of Science, and Google Scholar using combinations of the following keywords: arbovirus, Crimean-Congo hemorrhagic fever, CCHF, chikungunya, dengue, Rift Valley fever, West Nile, yellow fever, Zika, epidemiology, seroprevalence, EMRO, Eastern Mediterranean, Middle East, Africa, Asia. Reference lists of eligible articles were hand-searched for additional relevant studies. Original research articles published in English assessing arbovirus seroprevalence or viral detection in human populations in EMRO member states were included. Review articles, case reports, animal studies, studies from non-EMRO countries, and studies published before 2014 were excluded. The following information was extracted from eligible studies: first author, year of publication, country, sample size, participant characteristics, arbovirus(es) tested, diagnostic methods, and key seroprevalence or viral detection results.
Results
Crimean-Congo hemorrhagic fever (CCHF)
Virology
CCHF, a zoonotic viral hemorrhagic ailment, has established a substantial presence across diverse regions, including Asia, Africa, Eastern Europe, and the Middle East. CCHFV is classified under the Bunyaviridae family and the Nairovirus genus [20]. This organism's genome is made up of three segments: small (S), medium (M), and large (L). The L segment encodes the RNA-dependent RNA polymerase (RdRp), while the S and M segments encode the nucleocapsid and glycoprotein structural proteins, respectively [21]. The incidence of CCHFV-related diseases primarily afflicts individuals engaged in occupations such as ranching, farming, butchery, and slaughterhouses, with transmission occurring through occupational contact with infected animal blood and tissues, tick bites, and nosocomial infections [20], [22]. A diverse range of animals, including cattle, dromedaries, goats, sheep, and select reptiles and birds, can host the CCHFV. Ticks, notably Hyalomma marginatum and Hylomma rufipes, serve as both reservoirs and vectors for the virus [23]. Figure 1 [Fig. 1] illustrates the geographical map of the distribution of CCHFV cases.
Figure 1: Geographical map of the distribution of CCHFV cases as reported in various studies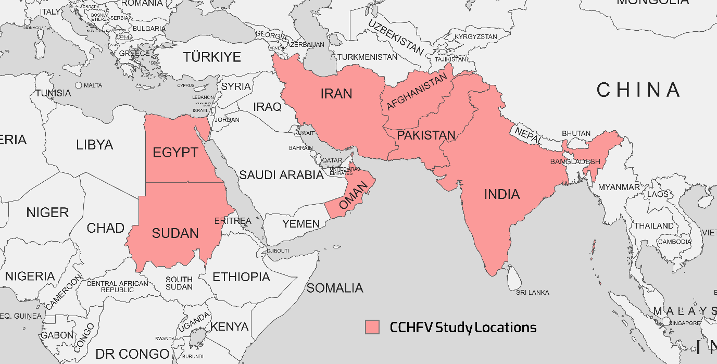
Diagnostics
The prevailing diagnostic method for detecting CCHFV in patient samples involves polymerase chain reaction (PCR) and reverse transcription-polymerase chain reaction (RT-PCR), a pivotal tool for virus detection. Rahden et al. [24], Sahak et al. [25], Shahbazi et al. [20], Todd et al. [26] and Khurshid et al. [27] used ELISA methods; Ahmed et al. [28], Habibzadeh et al. [22] and Umair et al. [21] used RT-PCR methods for detection of CCHF.
Clinical aspects
A majority of CCHFV infections remain subclinical or asymptomatic (approximately 90% of cases), while the fatality risk escalates to 10–40% when symptomatic manifestations occur. Common initial symptoms encompass headaches, myalgia, joint pain, elevated temperature, chills, diarrhea, nausea, and vomiting, often presenting mildly. A small minority of cases exhibit severe symptoms characterized by an abrupt onset, rapid onset of hemorrhaging, and significant hemorrhagic complications. The initial isolation of CCHFV from ticks in Kenya in 1975 marked a crucial milestone [20], [22], [23], [29]. The therapeutic options available to individuals afflicted with CCHF are primarily centered on supportive care, complemented by antiviral medication, notably Ribavirin [29].
Epidemiology
Reports of CCHFV infections extend to EMRO nations, including Iran, Pakistan, India, Afghanistan, Sudan, and Oman. Table 1 [Tab. 1] summarizes seroprevalence studies detecting CCHFV antibodies or viral RNA published between 2015–2023 in countries endemic for the disease in the EMRO. Reported CCHFV seroprevalence ranged widely from 0.4% in Iran [20] to as high as 78% in Sudan [28]. Overall, countries in the region with the highest pooled CCHFV exposure were Afghanistan (mean 33.5%, range 4.1–50.7%) and Sudan (mean 20.7%, range 1.47–78%). Significant variability was noted even within the same country. This may be attributable to differences in geographic locales, seasons, climates, diagnostic methods, or study populations. Additional longitudinal investigations are warranted to clarify CCHFV epidemiology and risk factors for human infection across the Middle East, Africa, and Asia. Improved surveillance and control measures are needed to mitigate the morbidity and mortality caused by this neglected viral hemorrhagic fever. Both RT-PCR and ELISA demonstrated the ability to detect the virus. In some instances, RT-PCR identified more positive samples than paired ELISA testing, suggesting it may be a more sensitive diagnostic technique for this particular virus. However, ELISA may be more feasible in resource-limited settings.
Table 1: Summary of the CCHFV seroprevalence studies in the EMRO region, 2015–January 2024 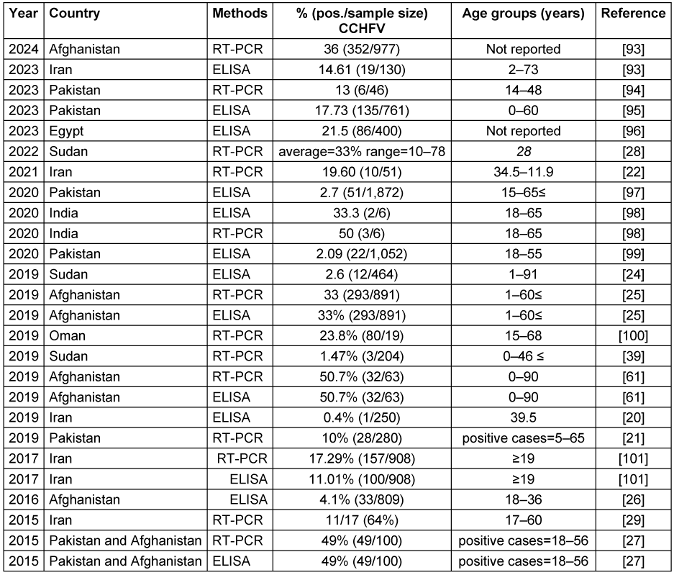
Chikungunya virus (CHIKV)
Virology
CHIKV, a member of the Togaviridae family in the alphavirus genus, is classified as an arbovirus due to its transmission by arthropod vectors. CHIKV is characterized by its enveloped nature, single-stranded positive-sense RNA genome, and affiliation with the flavivirus group. This virus primarily afflicts individuals residing in tropical and subtropical regions of Africa and Asia, mainly spreading through the Aedes species of mosquitoes, namely Aedes aegypti and Aedes albopictus, which serve as the vectors in this geographical context [15], [30]. As early as 1983, there were reports of CHIKV circulating among rodents in Pakistan, although human cases were few. In the midst of a dengue outbreak in Lahore in 2011, it was found that some patients also had antibodies to CHIKV. By 2016, CHIKV had surfaced in Karachi, and an outbreak was eventually declared once local transmission was confirmed [31].
Diagnostics
The CHIKV can be diagnosed through various methods, such as virus isolation from clinical specimens such as serum or plasma by inoculation into susceptible cell lines (e.g., Vero, BHK-21); this is considered the gold standard, but it is time-consuming. Molecular methods like RT-PCR and real-time RT-PCR are suitable for rapid and sensitive detection of CHIKV RNA during the acute phase. Serological methods – such as ELISAs and rapid tests for detecting CHIKV-specific IgM and IgG antibodies in serum or plasma samples, and antigen detection using rapid diagnostic tests that detect CHIKV antigens in clinical specimens – are useful in resource-limited settings or outbreaks [32], [33].
Clinical aspects
The initial clinical presentation of CHIKV infection often resembles other tropical fever illnesses. What sets CHIKV apart is the debilitating arthralgia it induces. This condition frequently affects multiple joints and exhibits a bilateral pattern. Additional clinical manifestations include conjunctivitis, asthenia, peripheral edema, headaches, and gastrointestinal disturbances. Moreover, CHIKV infection can lead to severe complications such as sepsis and the involvement of vital organs such as the heart, kidneys, and nervous system. Dermatological and ophthalmic manifestations are also not uncommon. Furthermore, CHIKV infection can have persistent consequences, including chronic pain, rheumatic symptoms, depression, and disturbances in mood and sleep patterns [34]. Without laboratory testing, cases of CHIK are often underreported because its signs and symptoms match those of dengue. Both diseases are transmitted by the same insect vectors [29].
Epidemiology
Historically, the first documented CHIKV outbreak occurred in 1952 on the Makonde Plateau, situated on the border between Mozambique and Tanzania [35]. Subsequently, the virus has reemerged in various regions, including Africa, Indian Ocean islands, South and Southeast Asia, the Americas, the Pacific, and Europe. This resurgence can be attributed, in part, to human migration across the Atlantic and Pacific oceans [36]. The geographical map of the distribution of CHIKV cases is shown in Figure 2 [Fig. 2].
Figure 2: The geographical map of the distribution of CHIKV cases as reported in various studies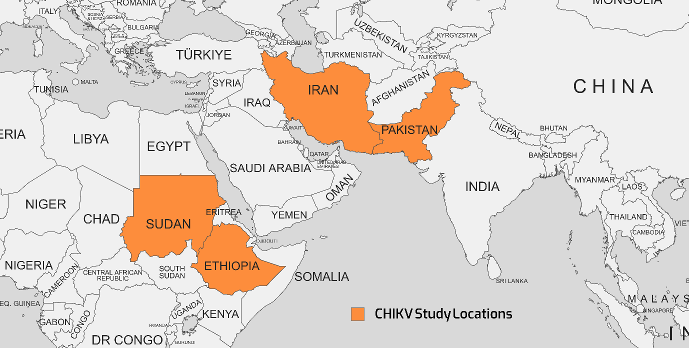
Findings from studies between 2016–2021 that used RT-PCR and ELISA methods are presented in Table 2 [Tab. 2]. Reported viral seroprevalence varied widely. In Pakistan, RT-PCR results ranged from 15.98% to 50% between 2018–2021. In Sudan, the seroprevalence of RT-PCR was as high as 84.5%, but as little as 0% in different study cohorts from 2019–2021. Both ELISA and RT-PCR detected the virus, but RT-PCR identified more positive cases in several studies, suggesting it may be a more sensitive test. The significant variability in viral prevalence, even within the same country and year, indicates localized epidemics and seasonal fluctuations rather than homogeneous endemic transmission. Herd immunity, circulating strains, geography, climate, social factors like crowding, and study sampling likely all contribute to the observed differences. Overall, the results confirm the circulation of this important virus in multiple Eastern Mediterranean nations in recent years. Ongoing surveillance and analysis of geographic and temporal patterns are essential to target public health interventions, e.g., vaccination, vector control, and behavior change communication, to the most affected populations. Improved reporting to regional databases would also help characterize the shifting epidemiology of this virus. Notably, Sudan reported both the highest and lowest seroprevalence rates. Furthermore, co-infection involving chikungunya and dengue viruses has been documented in two studies in Iran between 2021 and 2020, respectively [37], [38]. Detailed information regarding these co-infections is provided in Table 3 [Tab. 3], which separately discusses the epidemiology of the dengue virus.
Table 2: Summary of the CHIKV seroprevalence studies in the EMRO region, 2016–2021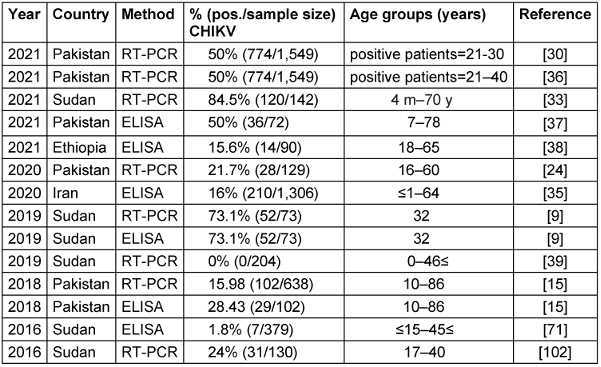
Table 3: Summary of the DENV seroprevalence studies in the EMRO region, 2014–2023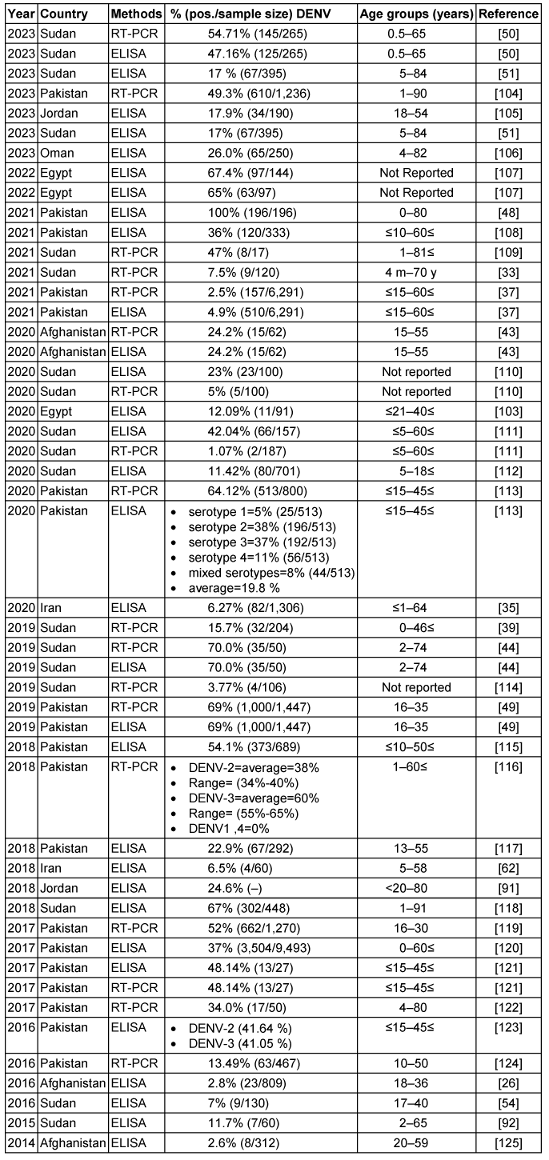
Dengue fever virus (DENV)
Virology
DENV is a mosquito-borne viral disease caused by one of four closely related dengue virus serotypes (DENV1–4) of the class flavivirus and family flaviviridae, which likewise incorporates chikungunya, yellow fever, and Zika viruses [39], [40]. Clinical manifestations of dengue infection vary, ranging from a self-limiting illness that resembles the flu to the severe lethal form of dengue hemorrhagic fever or dengue shock syndrome (DSS) [39]. Aedes (A.) aegypti and A. albopictus mosquitoes are the primary vectors of dengue in EMRO countries. Rapid urbanization and increased population density have promoted vector breeding in artificial containers. Climate factors also influence the spatial and seasonal distribution of vectors. Effective surveillance and targeted control of aedes mosquitoes are critical to prevent transmission [41]. The reservoir of this virus includes monkeys [42], and its vector is the A. Aegypti mosquito [39].
Diagnostics
The diagnosis of DENV infection can be achieved through various methods, including molecular techniques such as RT-PCR and qRT-PCR, which are highly sensitive and specific for detecting DENV RNA during the acute phase of infection. Virus isolation through inoculation of clinical samples into mosquito cell lines or mammalian cell cultures is time-consuming and requires specialized facilities. Serological assays, such as ELISAs and rapid diagnostic tests (RDTs) for detecting DENV-specific antibodies (IgM and IgG) in serum or plasma samples, are useful in the later stages of infection. Antigen detection assays, including non-structural protein 1 (NS1) capture ELISAs and RDTs, can detect DENV antigens during the early phase of illness [43], [44].
Clinical aspects
About 75% of dengue infections are asymptomatic, and 5% of cases are severe. If appropriately treated, the case:fatality ratio for severe dengue can be as low as 0.1% and as high as 10%. Both symptomatic and asymptomatic individuals can spread DENV to mosquitoes that bite them throughout the seven-day infectivity period, since they are both viremic. The intrinsic incubation period of DENV in humans, which is the time before symptoms appear, is 3–14 days [45].
Epidemiology
Many variables, such as human population expansion, density, migration, cross-border commerce and travel, water shortages and inadequate water storage, as well as global climate change, might contribute to the spread of dengue [46]. Two of the most prevalent mosquito-borne diseases in Southeast Asia, the Western Pacific region, and the United States are DF and dengue hemorrhagic fever (DHF). Iran has always been vulnerable to DENV because of its geographic location and proximity to DENV-endemic countries such as Afghanistan and Pakistan [47]. Since 1799, there have been reports of dengue outbreaks in Egypt, specifically in the governorates of Cairo and Alexandria [48]. The lack of authorized dengue treatments or vaccinations puts the world's population at risk of illness during a pandemic [40]. Dengue is widespread in EMRO countries, with epidemic activity reported in most countries in recent years. It is estimated that 100 million dengue infections and over 20,000 deaths related to severe dengue occur in the region annually [49]. Figure 3 [Fig. 3] shows the geographical map of the DENV case distribution.
Figure 3: Geographical map of the DENV case distribution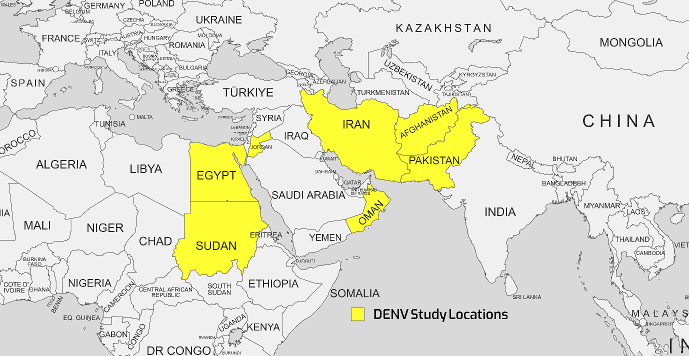
Table 3 [Tab. 3] summarizes over 30 studies on the seroprevalence of DENV in EMRO countries between 2014 and 2023. The main diagnostic methods used were ELISA serology testing and RT-PCR to detect viral RNA. The prevalence rates varied widely across the different studies and countries. Reported DENV seroprevalence varied widely, ranging from 2.5% to 100% in Pakistan [50].
In Pakistan, multiple studies over the years consistently found some of the highest DENV exposure, with positive rates reaching 69% by RT-PCR [51]. Serotypes DENV-2 and DENV-3 predominated. Sudan also exhibited high DENV seroprevalence, up to 70% in certain locales [46]. Other countries had more moderate exposure – around 17–26% in recent studies from Jordan, Egypt, and Oman. Afghanistan and Iran showed lower DENV seropositivity, less than 5% in the included investigations. Significant differences were observed between ELISA and PCR-based assessments, with PCR detecting acute infection and ELISA measuring historical exposure. Additional factors like geographic region, population, season, sample size, and epidemic years likely contributed to variability between studies. Overall, the table highlights DENV as an important public health threat in the Middle East and southern Asia. Improved surveillance, vector control, and vaccines are needed to prevent DENV epidemics and reduce the disease burden in endemic countries. In summary, the dengue virus remains an important public health threat with evidence of ongoing endemic transmission in parts of the EMRO. Continued surveillance and standardized methods are needed to elucidate the epidemiology fully.
Rift Valley fever virus (RVF)
Virology
RVF, a mosquito-borne disease caused by the RVFV (genus Phlebovirus, family Bunyaviridae), has caused epidemics in Africa and, more recently, the Arabian Peninsula, that are affecting both livestock and humans [52]. The virus, transmissible through mosquito bites, exposure to infected animal fluids, or consumption of raw milk from infected animals, was documented outside Africa in Saudi Arabia and Yemen in 2000, potentially due to imported infected animals [53]. Additionally, human and vector mosquito populations can continue transmitting RVFV vertically [54]. Mosquitoes are the primary RVF vectors, especially Aedes and Culex species, which proliferate after heavy rains and floods [55].
Diagnostics
DENV infection can be diagnosed using various methods, including nucleic acid amplification tests (NAATs) and IgM antibody testing. NAATs are the preferred method for patients with a clinically compatible illness who live in or recently traveled to a disease-endemic area. Laboratory confirmation can be made using RT-PCR or NS1 antigen. IgM detection is most useful for patients presenting more than one week after fever onset. Both ELISA and RT-PCR are approved as in vitro diagnostic tests [44], [56].
Clinical aspects
The recent outbreak presented a febrile hemorrhagic syndrome with liver and renal dysfunction, resulting in 882 confirmed cases and 124 deaths (possibly underreported). Travelers to endemic areas risk acquiring RVF [97]. RVF in humans usually manifests as a mild illness with influenza-like symptoms. However, in 1–3% of patients, the disease can progress to a severe condition characterized by hemorrhaging, potentially fatal encephalitis, liver necrosis, ocular disease, both internal and external bleeding, and other symptoms [57]. Pregnancy-related infections pose a significant risk to both the mother and fetus. Such infections can lead to fetal deformity, miscarriage, or preterm delivery.
Epidemiology
Emerging vector-borne diseases caused by pathogens such as ZIKV, WNV, Japanese encephalitis virus, Venezuelan equine encephalitis virus, malaria, brucellosis, and dengue can result in similar complications [52]. In nations where RVF is endemic, especially Sudan, which relies on the export of animals and animal products, RVF outbreaks pose substantial public health and economic risks [54]. RVF was initially identified among exotic sheep between Lake Naivasha and Lake Elmenteita in the Kenyan Rift Valley in 1910 and 1912 [58]. Later, in 1944, Smithburn, who was employed by the Yellow Fever Research Institute in Entebbe, Uganda, obtained two virus isolates from mosquitoes [59]. These mosquitoes, belonging to the Aedes tarsalis and the Eretmapodites spp., were collected from the untamed forest of Western Uganda [58], [60]. The seroprevalence rates reported vary considerably between the studies (Table 4 [Tab. 4]). In 2020, Ahmed et al. [54] tested samples using RT-PCR and found an average seroprevalence of 33.3% with a wide range from 0.1% to 99.2%. This indicates substantial geographic variation in viral circulation within Sudan in that year. In contrast, two studies from 2019 found a much lower seroprevalence of 6.8% [9] and even 0% seroprevalence [61]. Other factors like seasonality, study population characteristics, sample size, and assay methods could also contribute to the variability. Overall, the results confirm that this virus circulates in Sudan, but prevalence appears to fluctuate dramatically based on setting and period. Improved surveillance and standardized diagnostics are needed to characterize the epidemiology of this virus in Sudan fully. Risk factors for hot spots of infection require further study to support evidence-based control measures.
Table 4: Summary of the RVFV seroprevalence studies in the EMRO region, 2019–2020
West Nile virus (WNV)
Virology
WNV is a positive-sense, single-stranded RNA virus that is spread by mosquitoes and belongs to the family flaviviridae [62]. WNV is also the most frequently reported Culex-transmitted virus in Iran. Ornithophilic mosquitoes and migratory birds perpetuate the WNV enzootic cycle, but mammals (e.g., humans and horses) do not. Members of the genus Culex (C.), primarily the C. pipiens, C. univittatus, C. antennatus, and C. vishnui complex, are the main mosquito vectors [16], [63]. Although the primary mode of WNV transmission to humans is through mosquito bites, it can also spread in several other ways. These include blood transfusion, breastfeeding, laboratory occupational exposure, and organ transplants [62], [64].
Diagnostics
WNV diagnosis involves several methods. Clinicians typically consider WNV in patients with compatible symptoms who live in or recently traveled to an endemic area within two weeks before symptom onset. The preferred laboratory method is testing serum or cerebrospinal fluid (CSF) for WNV-specific IgM antibodies. These antibodies are usually detectable 3–8 days after symptom onset and persist for 30 to 90 days. However, cross-reactivity with other flaviviruses can complicate interpretation. IgG antibodies indicate previous infection, while plaque-reduction neutralization tests (PRNTs) can determine the specific infecting flavivirus. Molecular tests (viral cultures and RT-PCR) can confirm infection, but their likelihood of detecting WNV is low. Immunohistochemistry (IHC) detects WNV antigens in tissue samples. State public health laboratories or the CDC can perform these tests [65], [66].
Clinical aspects
Most WNV infections are asymptomatic, but in 20% of instances, the infection causes West Nile Fever (WNF), and in fewer than 1% of cases, it causes acute West Nile Neuroinvasive Disease (WNND) [63]. In 80% of patients, the infection is asymptomatic. Without this, infected individuals may develop a severe febrile illness. The severity of this illness can range from a self-limited infection to encephalitis, which can result in long-term impairment and death in 10% of neurologic cases [67].
Epidemiology
WNV isolates from various geographical locations have been divided into up to nine lineages by phylogenetic analysis. Outbreaks of major viral encephalitis in humans have been associated with lineages 1, 2, and 5. WNV infections have been reported both in Pakistan and in neighboring countries such as Iran, China, and India [62]. The WNV, first discovered in 1937 in the West Nile region of Uganda, has since been linked to epidemics across Africa, the Middle East, especially Egypt, and Western Asia. Outbreaks have been reported in North America and Europe since the 1990s [64], [68]. Table 5 [Tab. 5] summarizes findings from 8 studies between 2014–2023 that investigated the seroprevalence of a specific virus in several Eastern Mediterranean countries, including Pakistan, Sudan, Iran, Jordan, Lebanon, and Qatar. Reported WNV seroprevalence ranged from 0.5% in Afghanistan [69] to 28.8% in Tunisia [70]; Figure 4 [Fig. 4] shows the geographical map of the WNV case distribution. In general, the highest WNV exposure was found in Tunisia, with around 10-30% positive rates. Lebanon, Jordan, Iran, and Pakistan followed with moderate WNV seroprevalence of 1.9–20.6% in recent studies. The lowest seropositivity was observed in Afghanistan and Qatar (0.5–3.3%). Significant differences were noted between PCR and ELISA results, since PCR detects current WNV infection while ELISA measures historical exposure. Geographic region, seasonality, study population, and sample size likely contributed to variability between studies.
Table 5: Summary of the WNV seroprevalence studies in the EMRO region, 2014–2023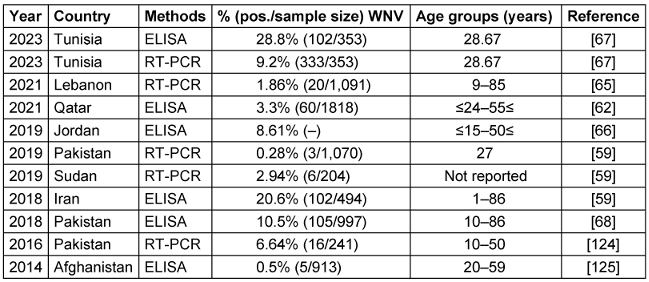
Figure 4: Geographical map of the distribution of WNV cases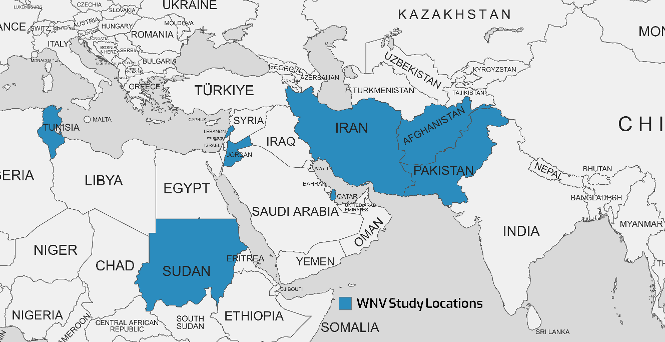
Overall, Table 5 [Tab. 5] indicates that WNV remains an endemic public health concern in the region. Additional surveillance is warranted, especially in Tunisia, which has very high infection rates. Improved mosquito control and personal protective measures are needed to reduce WNV transmission to humans. WNV vaccination may benefit high-risk groups in endemic areas. Improved reporting to WHO and other regional databases would strengthen the evidence base for targeted public health interventions.
Yellow Fever Virus (YFV)
Virology
YFV is a zoonotic flavivirus with a positive-sense, single-stranded RNA genome, which is prevalent in the tropics of South America and Africa. The Haemagogus, Sabethes, and Aedes genera of mosquitoes are the vectors responsible for maintaining and transmitting the YFV reservoir to humans. This process is known as the “sylvatic cycle” and often occurs when humans encroach upon the native habitats of monkeys. Once YFV has infected humans, it is disseminated through an “urban cycle” by a new vector, the anthropophilic A. aegypti mosquito [71], [72], [73].
Diagnostics
YFV can be detected using various methods, including real-time RT-PCR during the initial viremic phase, virus isolation in cell cultures or inoculation in mice, serological assays like ELISA and IIF, and immunohistochemistry in fatal cases. Molecular techniques such as RT-PCR are used to detect viral RNA in blood samples, while isolating the virus requires biosafety l Level 2 containment. However, these methods can exhibit cross-reactivity with other flaviviruses. Immunohistochemistry can provide a definitive diagnosis in fatal cases. The PRNT is considered the gold standard for confirming YFV infection due to its high specificity [74], [75].
Clinical aspects
The WHO defines a YF-suspected case as any person who exhibits an acute febrile fever and the development of jaundice within 14 days after the onset of symptoms [76]. Clinical manifestations range from a mild flu-like illness to hemorrhagic fever, with a possible case fatality rate of 20% to 50%. Usually, those who visit or work in jungle environments are at risk of unintentional exposure to the virus [72]. Previous studies have demonstrated the persistent presence of RNA from various flaviviruses, including YFV, dengue virus, and ZIKV, in urine or saliva [77].
Epidemiology
Nigeria, Uganda, Ghana, Chad, Guinea, the Republic of the Congo, and Angola are African countries that have recently experienced YF outbreaks. Since the 1960s, numerous YF outbreaks in Ethiopia have resulted in the deaths of over 30,000 people in the southern region. An YF outbreak re-emerged in the South Omo Zone of southern Ethiopia in 2013, leading to many fatalities [71], [76]. The seroprevalence rates varied considerably across the studies, ranging from 0% in 2019 to 46% in Sudan in 2016 [78]. The 0% seroprevalence found with RT-PCR testing in Sudan in 2019 indicates that the virus may have been absent or circulating at extremely low levels in the particular geographic area and period sampled. This contrasts sharply with the 46% seropositivity rate found just three years prior in Sudan in 2016, signaling an active epidemic at that time. The 2.9% seroprevalence found in Ethiopia in 2021 by Asebe et al. [79] suggests more limited transmission but still with evidence of circulation, as shown in Table 6 [Tab. 6]. Figure 5 [Fig. 5] depicts the geographical map of the YFV case distribution.
Table 6: Summary of the YFV seroprevalence studies in the EMRO region, 2016–2021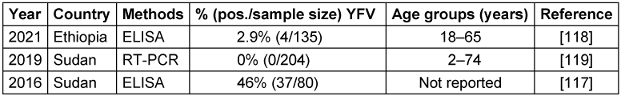
Figure 5: A geographical map of the distribution of YFV cases as reported in various studies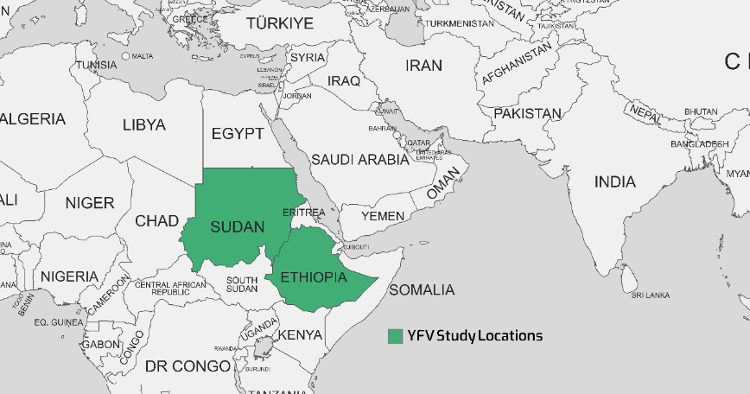
Zika virus (ZIKV)
Virology
ZIKV is an emerging pathogen of significant public health concern. While most infections result in self-limiting symptoms, the recent outbreak has been linked to an increased incidence of congenital anomalies, including microcephaly. ZIKV is primarily transmitted by Aedes mosquitoes, particularly A. aegypti and A. albopictus. Structurally, ZIKV belongs to the flavivirus family, sharing closer genetic ties with DENV and YFV and more distantly with WNV. Its genome consists of a single-stranded, positive-sense RNA encoding a polyprotein that undergoes cleavage into functional domains, including capsid, the precursor of the membrane (prM), and envelope (E), along with seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [80], [81].
Diagnostics
Zika virus diagnosis involves two main methods: direct detection of viral components and antibody-based tests. NAATs, particularly RT-PCR, are the gold standard for confirming an active infection, targeting viral RNA in bodily fluids. Serological assays such as ELISA detect antibodies against ZIKV, which is useful in later stages or in identifying past exposure. However, RT-PCR offers the most definitive diagnosis during the initial stages of ZIKV infection, as it may show cross-reactivity with other flaviviruses [82], [83].
Clinical aspects
Clinical symptoms of ZIKV infection include low-grade fever, maculopapular rash, arthralgia, and non-purulent conjunctivitis. Despite extensive research, no specific antiviral therapies or vaccines are available for ZIKV, making it a significant global health challenge. It can be passed from a mother to a fetus during pregnancy and can also be transmitted through sexual contact [84]. ZIKV can spread in several ways, including urine, mosquitoes bite, sexual intercourse, blood transfusions, and transmission from mother to fetus. The incubation period for ZIKV is around one week, after which it can cause disorders such as Guillain-Barré syndrome (GBS), microcephaly, and symptoms such as joint pain, skin rash, headache, fever, and conjunctivitis. Some people may also experience vomiting, diarrhea, eye redness, lethargy, and edema [85], [86].
Epidemiology
ZIKV was first isolated from Rhesus monkeys in 1947 and then from the Aedes mosquito in 1948 in the Zika forest of Uganda. The first documented case of human infection occurred in Nigeria in 1954. The ZIKV pandemic began in Brazil and spread to 60 different nations in 2015. A significant outbreak was observed in Brazil in 2016 [71], [63], [87]. Additionally, mosquitoes transmit ZIKV within a sylvatic ecosystem during an enzootic cycle that involves non-human primates, specifically monkeys. These infected mosquitoes can spread the disease to humans during an epidemic cycle. People infected with ZIKV often recover spontaneously; thus, they may not require specific treatment. While the demand for safe and effective ZIKV vaccines is global, there are no approved and readily accessible antiviral medications that can effectively prevent or cure the infection [86], [88]. A. aegypti and A. albopictus mosquitoes are likely Zika vectors in EMRO, given their role in transmission elsewhere. These species’ wide distribution in urban areas where dense populations coexist raises concern for explosive Zika outbreaks, like those seen in the Americas in 2015–2016. Targeting Aedes through vector control and personal protection are the first lines of defense against potential Zika emergence [71], [63]. Table 7 [Tab. 7] presents the results of studies conducted on the ZIKV that showed that seroprevalence rates ranged from 0% in two studies in Sudan and Iran in 2018–2019 to as high as 62.7% in Sudan in 2018. The geographical map of the ZIKV case distribution is shown in Figure 6 [Fig. 6].
Table 7: Summary of the ZIKV seroprevalence studies in the EMRO region, 2018–2021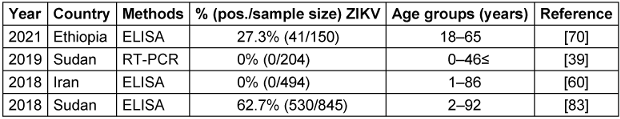
Figure 6: Geographical map of the ZIKV cases distribution as reported in various studies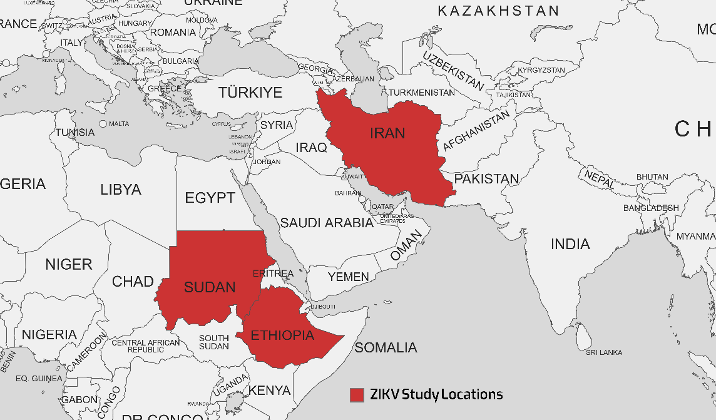
Surveillance, prevention and control measures
Surveillance systems play a crucial role in monitoring the spread of arboviral diseases and detecting outbreaks at an early stage. These systems rely on various methods, including passive case reporting from healthcare facilities, sentinel surveillance through selected health centers or hospitals, epidemiological investigations, and serological tests. Timely reporting of suspected cases allows public health authorities to initiate appropriate control measures, such as vector control activities, vaccination campaigns, etc. [89].
Prevention and control measures for arboviral diseases in the EMRO region involve a multi-faceted approach. These include vector control strategies such as reducing mosquito breeding sites, using insecticide-treated bed nets, and indoor residual spraying. Vaccination programs have also been implemented for certain arboviral diseases, such as dengue fever [130]. Vaccination is now seen as a viable future option by a significant portion of the world's population. Few arboviral vaccinations are available, but they constantly threaten human and animal health [1], [130]. Insect-specific viruses (ISVs) present new potential for vaccine development because they cannot reproduce in vertebrates or their cells [1].
Public health campaigns are regularly conducted to highlight the importance of personal protective measures such as wearing long sleeves and using insect repellents. In addition, healthcare professionals are provided with training programs on early diagnosis and case management. Arboviruses can survive in dormant mosquito eggs for months or even years before the rainy season causes the emergence of healthy but infected mosquito larvae. These viruses frequently take advantage of the longer life cycles of some tick species, surviving for years through the trans-stadial stages and reproducing at low rates [2].
Discussion
This review synthesizes evidence on the epidemiology of major arboviruses in the EMRO between 2014–2023. The data reveals a significant burden of viral diseases across the EMRO region, with varying prevalence rates observed for different pathogens. Notably, the CCHFV exhibits a wide range of prevalence, from 0.4% in Iran (2019) [20] to 64% in the same country (2015) [29], with an overall average of 33%. This highlights the need for robust prevention and control measures to combat CCHFV in the region.
Furthermore, the data underscores the alarming prevalence of DENV in certain areas, with Pakistan reporting a staggering 100% rate in 2021 [50]. The distribution of DENV serotypes also varies, with serotypes 2 and 3 being the most prevalent. This information is crucial for developing targeted interventions and vaccines against the circulating serotypes [90], [91].
WNV antibodies were commonly detected as well, pointing to extensive WNV transmission in the region [70]. RVFV and YFV were detected less frequently; however, the data was limited. Finally, ZIKV was found circulating in Sudan in 2018 at a high rate of 62.7%, indicating active transmission [85].
The significant variability in reported prevalence highlights the need for standardized methods and systematic surveillance within and between EMRO countries. Differences in geographic location, climate, seasons, study population, sample size, and assay techniques likely contributed to the observed ranges. However, clear hot spots of transmission are evident for some viruses like DENV in Pakistan.
Overall, while this review synthesizes recent evidence on arbovirus epidemiology in EMRO countries, the limitations may restrict the ability to fully characterize transmission patterns and risk factors across the region. Improved coordination and data sharing between regional public health authorities could strengthen the evidence base for targeted prevention and control measures. Given the significance of arboviruses and their proliferation in various regions worldwide, including countries in the EMRO region, there is a pressing need for research and preventative measures to curb their escalating spread. The majority of the detected DENV infections presented were accompanied by bleeding, indicative of dengue hemorrhagic fever. According to WHO guidelines, this is recognized as one of the most severe forms of the disease [39]. To facilitate readers’ understanding, a holistic view of the overall results is depicted in Figure 7 [Fig. 7], Figure 8 [Fig. 8], Figure 9 [Fig. 9], and Figure 10 [Fig. 10]. The majority of arbovirus studies were conducted in Pakistan and Sudan, with 35 studies, followed by Iran, with 18 studies. This suggests that Pakistan and Sudan are at the forefront of arbovirus research in the EMRO region. The remaining countries have conducted far fewer studies (Figure 7 [Fig. 7]).
Figure 7: Number of studies conducted in each country during 2014–2024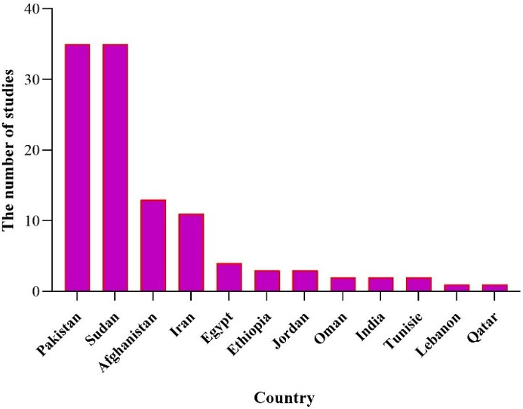
Figure 8: Number of viruses examined in each country during 2014–2024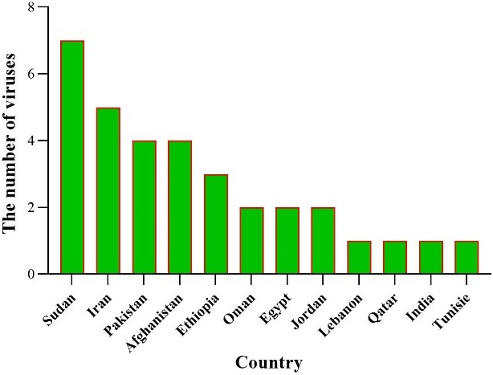
Figure 9: General Distribution of arbovirus detection by RT-PCR methods in the EMRO region 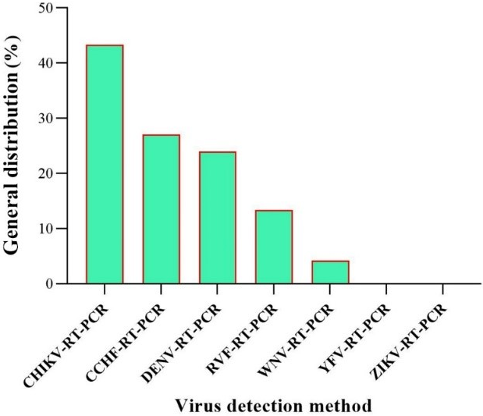
Figure 10: General distribution of arbovirus detection by ELISA methods in the EMRO region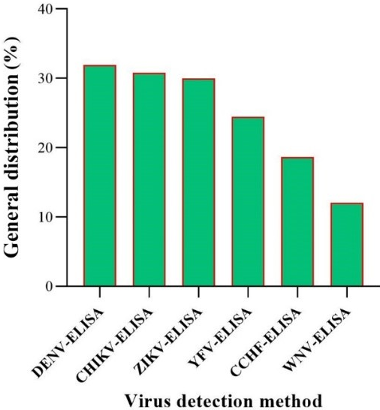
Sudan has examined the most viruses, followed by Iran, Pakistan, and Ethiopia. This suggests that Sudan has tested all seven viruses mentioned in this study within its own borders (Figure 8 [Fig. 8]).
The most common viruses detected were CHIKV, CCHF, DENV, RVF, WNV, YFV, ZIKV, and CCHF. This suggests that these viruses are of major public health importance in the EMRO region. WNV was 10.75% by ELISA and 2.93% by RT-PCR. In addition, the RT-PCR method failed to detect ZIKV and YFV, which could indicate that the ELISA test is more sensitive than RT-PCR in the case of these viruses (Figure 9 [Fig. 9]).
The highest detection rate is observed for DENV-ELISA at approximately 33%, while the lowest is for WNV-ELISA at about 12%. This figure underscores the varying prevalence of these viruses as detected by ELISA, highlighting the importance of continued monitoring and research (Figure 10 [Fig. 10]).
The EMRO region faces several challenges in addressing arboviral diseases. Limited resources, including funding, trained personnel, and laboratory capacity, can hinder surveillance efforts and timely response to outbreaks. The lack of well-established surveillance systems in some countries within the EMRO may result in underreporting or delayed detection of cases. Climate change is also a factor that can influence the spread of vectors and increase the risk of arboviral diseases [89].
Limitations
This review has some limitations that should be considered when interpreting the findings. First, we focused solely on evidence of arboviral antibodies or genome presence in human blood/serum samples. Studies detecting arboviruses in non-human hosts or vectors were excluded. Second, we restricted our search to publications from 2014 to January 2024 to examine current epidemiological patterns. Relevant studies before 2014 may have been missed. Third, full-text access was not available for some identified articles, which limited data extraction. Fourth, there was heterogeneity in study design, sampling methods, diagnostic techniques, and reporting formats between the included studies. Finally, the search was restricted to English language publications and thus relevant studies published in other languages may have been overlooked.
Conclusions
This study has provided a detailed overview of the prevalence of arboviruses within the EMRO, highlighting the significant public health challenge posed by these pathogens. Our findings indicate that arboviruses are increasingly becoming a cause for concern in this region due to factors such as climate change, urbanization, and increased human mobility. The data presented underscores the urgent need for enhanced surveillance systems to monitor arbovirus activity and the vectors responsible for their transmission. It is imperative that member states in the EMRO region collaborate to strengthen vector control measures and develop strategies to mitigate the risk of outbreaks. Public health initiatives must also focus on community education to raise awareness about the prevention of arboviral diseases. Furthermore, our research calls for the integration of arbovirus monitoring into national health systems, ensuring that resources are allocated efficiently to combat the spread of these diseases. The establishment of regional centers of excellence for arbovirus research can facilitate the sharing of knowledge and technical expertise, fostering a proactive approach to disease management and control.
In conclusion, the threat of arboviruses in the EMRO region is a multifaceted issue that requires a coordinated, multidisciplinary response. By adopting a holistic approach that encompasses surveillance, prevention, and research, the EMRO region can effectively address the challenges posed by arboviruses and safeguard the health of its populations.
Notes
Competing interests
The authors declare that they have no competing interests.
Authors’ ORCID
- Owliaee I: https://orcid.org/0000-0002-9695-4938
- Khaledian M: https://orcid.org/0000-0002-8123-7856
- Shojaeian A: https://orcid.org/0000-0002-1166-385X
- Jalilian FA: https://orcid.org/0000-0003-3134-1441
Funding
None.
References
[1] Pijlman GP. Enveloped virus-like particles as vaccines against pathogenic arboviruses. Biotechnol J. 2015 May;10(5):659-70. DOI: 10.1002/biot.201400427[2] Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg Microbes Infect. 2015 Mar;4(3):e18. DOI: 10.1038/emi.2015.18
[3] Centers for Disease Control and Prevention. Arbovirus Catalog – Virus Selection. Atlanta: CDC. Available from: https://wwwn.cdc.gov/arbocat/
[4] Gubler DJ. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Trop Med Health. 2011 Dec;39(4 Suppl):3-11. DOI: 10.2149/tmh.2011-S05
[5] Girard M, Nelson CB, Picot V, Gubler DJ. Arboviruses: A global public health threat. Vaccine. 2020 May;38(24):3989-94. DOI: 10.1016/j.vaccine.2020.04.011
[6] Higuera A, Ramírez JD. Molecular epidemiology of dengue, yellow fever, Zika and Chikungunya arboviruses: An update. Acta Trop. 2019 Feb;190:99-111. DOI: 10.1016/j.actatropica.2018.11.010
[7] Rosenberg R, Beard CB. Vector-borne infections. Emerg Infect Dis. 2011 May;17(5):769-70. DOI: 10.3201/eid1705.110310
[8] Liang G, Li X, Gao X, Fu S, Wang H, Li M, Lu Z, Zhu W, Lu X, Wang L, Cao Y, He Y, Lei W. Arboviruses and their related infections in China: A comprehensive field and laboratory investigation over the last 3 decades. Rev Med Virol. 2018 Jan;28(1). DOI: 10.1002/rmv.1959
[9] Mohamed N, Magzoub M, Mohamed REH, Aleanizy FS, Alqahtani FY, Nour BYM, Alkarsany MMS. Prevalence and identification of arthropod-transmitted viruses in Kassala state, Eastern Sudan. Libyan J Med. 2019 Dec;14(1):1564511. DOI: 10.1080/19932820.2018.1564511
[10] Argondizzo APC, Silva D, Missailidis S. Application of Aptamer-Based Assays to the Diagnosis of Arboviruses Important for Public Health in Brazil. Int J Mol Sci. 2020 Dec;22(1). DOI: 10.3390/ijms22010159
[11] Varghese J, De Silva I, Millar DS. Latest Advances in Arbovirus Diagnostics. Microorganisms. 2023 Apr;11(5). DOI: 10.3390/microorganisms11051159
[12] Azari-Hamidian S, Norouzi B, Harbach RE. A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Trop. 2019 Jun;194:106-22. DOI: 10.1016/j.actatropica.2019.03.019
[13] Paksa A, Azizi K, Yousefi S, Dabaghmanesh S, Shahabi S, Sanei-Dehkordi A. First report on the molecular phylogenetics and population genetics of Aedes aegypti in Iran. Parasit Vectors. 2024 Feb;17(1):49. DOI: 10.1186/s13071-024-06138-3
[14] Caragata EP, Tikhe CV, Dimopoulos G. Curious entanglements: interactions between mosquitoes, their microbiota, and arboviruses. Curr Opin Virol. 2019 Aug;37:26-36. DOI: 10.1016/j.coviro.2019.05.005
[15] Barr KL, Khan E, Farooqi JQ, Imtiaz K, Prakoso D, Malik F, Lednicky JA, Long MT. Evidence of Chikungunya Virus Disease in Pakistan Since 2015 With Patients Demonstrating Involvement of the Central Nervous System. Front Public Health. 2018;6:186. DOI: 10.3389/fpubh.2018.00186
[16] Bakhshi H, Mousson L, Moutailler S, Vazeille M, Piorkowski G, Zakeri S, Raz A, de Lamballerie X, Dinparast-Djadid N, Failloux AB. Detection of arboviruses in mosquitoes: Evidence of circulation of chikungunya virus in Iran. PLoS Negl Trop Dis. 2020 Jun;14(6):e0008135. DOI: 10.1371/journal.pntd.0008135
[17] Kuno G, Chang GJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005 Oct;18(4):608-37. DOI: 10.1128/CMR.18.4.608-637.2005
[18] Eybpoosh S, Fazlalipour M, Baniasadi V, Pouriayevali MH, Sadeghi F, Ahmadi Vasmehjani A, Karbalaie Niya MH, Hewson R, Salehi-Vaziri M. Epidemiology of West Nile Virus in the Eastern Mediterranean region: A systematic review. PLoS Negl Trop Dis. 2019 Jan;13(1):e0007081. DOI: 10.1371/journal.pntd.0007081
[19] Madewell ZJ. Arboviruses and Their Vectors. South Med J. 2020 Oct;113(10):520-3. DOI: 10.14423/SMJ.0000000000001152
[20] Shahbazi N, Firouz SK, Karimi M, Mostafavi E. Seroepidemiological survey of Crimean-Congo haemorrhagic fever among high-risk groups in the west of Iran. J Vector Borne Dis. 2019;56(2):174-7. DOI: 10.4103/0972-9062.263720
[21] Umair M, Khurshid A, Alam MM, Akhtar R, Salman M, Ikram A. Genetic diversity and phylogenetic analysis of Crimean-Congo Hemorrhagic Fever viruses circulating in Pakistan during 2019. PLoS Negl Trop Dis. 2020 Jun;14(6):e0008238. DOI: 10.1371/journal.pntd.0008238
[22] Habibzadeh S, Mohammadshahi J, Bakhshzadeh A, Moradi-Asl E. The First Outbreak of Crimean-Congo Hemorrhagic Fever Disease in Northwest of Iran. Acta Parasitol. 2021 Sep;66(3):1086-8. DOI: 10.1007/s11686-021-00342-2
[23] Temur AI, Kuhn JH, Pecor DB, Apanaskevich DA, Keshtkar-Jahromi M. Epidemiology of Crimean-Congo Hemorrhagic Fever (CCHF) in Africa-Underestimated for Decades. Am J Trop Med Hyg. 2021 Apr;104(6):1978-90. DOI: 10.4269/ajtmh.20-1413
[24] Rahden P, Adam A, Mika A, Jassoy C. Elevated Human Crimean-Congo Hemorrhagic Fever Virus Seroprevalence in Khashm el Girba, Eastern Sudan. Am J Trop Med Hyg. 2019 Jun;100(6):1549-51. DOI: 10.4269/ajtmh.18-0977
[25] Sahak MN, Arifi F, Saeedzai SA. Descriptive epidemiology of Crimean-Congo Hemorrhagic Fever (CCHF) in Afghanistan: Reported cases to National Surveillance System, 2016-2018. Int J Infect Dis. 2019 Nov;88:135-40. DOI: 10.1016/j.ijid.2019.08.016
[26] Todd CS, Mansoor GF, Buhler C, Rahimi H, Zekria R, Fernandez S, Mikhail AF, Scott PT, Yingst SL. Prevalence of Zoonotic and Vector-Borne Infections Among Afghan National Army Recruits in Afghanistan. Vector Borne Zoonotic Dis. 2016 Aug;16(8):501-6. DOI: 10.1089/vbz.2015.1921
[27] Khurshid A, Hassan M, Alam MM, Aamir UB, Rehman L, Sharif S, Shaukat S, Rana MS, Angez M, Zaidi SS. CCHF virus variants in Pakistan and Afghanistan: Emerging diversity and epidemiology. J Clin Virol. 2015 Jun;67:25-30. DOI: 10.1016/j.jcv.2015.03.021
[28] Ahmed A, Ali Y, Salim B, Dietrich I, Zinsstag J. Epidemics of Crimean-Congo Hemorrhagic Fever (CCHF) in Sudan between 2010 and 2020. Microorganisms. 2022 Apr;10(5). DOI: 10.3390/microorganisms10050928
[29] Karlberg H, Sharifi-Mood B, Mousavi-Jazi M, Dilcher M, Lindegren G, Mardani M, Bereskly S, Weidmann M, Mirazimi A. Molecular and serological findings in suspected patients with Crimean-Congo hemorrhagic fever virus in Iran. J Med Virol. 2015 Apr;87(4):686-93. DOI: 10.1002/jmv.24106
[30] Badar N, Ikram A, Salman M, Alam MM, Umair M, Arshad Y, Mushtaq N, Mirza HA, Ahad A, Farooq U, Yasin MT, Qazi J. Chikungunya virus: Molecular epidemiology of nonstructural proteins in Pakistan. PLoS One. 2021;16(12):e0260424. DOI: 10.1371/journal.pone.0260424
[31] Badar N, Salman M, Ansari J, Aamir U, Alam MM, Arshad Y, Mushtaq N, Ikram A, Qazi J. Emergence of Chikungunya Virus, Pakistan, 2016-2017. Emerg Infect Dis. 2020 Feb;26(2):307-10. DOI: 10.3201/eid2602.171636
[32] Andrew A, Navien TN, Yeoh TS, Citartan M, Mangantig E, Sum MSH, Ch'ng ES, Tang TH. Diagnostic accuracy of serological tests for the diagnosis of Chikungunya virus infection: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2022 Feb;16(2):e0010152. DOI: 10.1371/journal.pntd.0010152
[33] Bower H, El Karsany M, Adam AAAH, Idriss MI, Alzain MA, Alfakiyousif MEA, Mohamed R, Mahmoud I, Albadri O, Mahmoud SAA, Abdalla OI, Eldigail M, Elagib N, Arnold U, Gutierrez B, Pybus OG, Carter DP, Pullan ST, Jacob ST, Abdallah TM, Gannon B, Fletcher TE. "Kankasha" in Kassala: A prospective observational cohort study of the clinical characteristics, epidemiology, genetic origin, and chronic impact of the 2018 epidemic of Chikungunya virus infection in Kassala, Sudan. PLoS Negl Trop Dis. 2021 Apr;15(4):e0009387. DOI: 10.1371/journal.pntd.0009387
[34] Meraj L, Saleem J, Manzoor S, Ashfaq A, Khurram M. First report of Chikungunya fever in Rawalpindi, Pakistan. East Mediterr Health J. 2020 Jun;26(6):744-7. DOI: 10.26719/emhj.19.095
[35] Tavakoli F, Rezaei F, Shafiei-Jandaghi NZ, Shadab A, Mokhtari-Azad T. Seroepidemiology of dengue and chikungunya fever in patients with rash and fever in Iran, 2017. Epidemiol Infect. 2020 Feb;148:e42. DOI: 10.1017/S0950268820000114
[36] Badar N, Ikram A, Salman M, Alam MM, Umair M, Arshad Y, Mushtaq N, Mirza HA, Ahad A, Yasin MT, Qazi J. Epidemiology of Chikungunya virus isolates 2016-2018 in Pakistan. J Med Virol. 2021 Nov;93(11):6124-31. DOI: 10.1002/jmv.26957
[37] Raza FA, Javed H, Khan MM, Ullah O, Fatima A, Zaheer M, Mohsin S, Hasnain S, Khalid R, Salam AA. Dengue and Chikungunya virus co-infection in major metropolitan cities of provinces of Punjab and Khyber Pakhtunkhwa: A multi-center study. PLoS Negl Trop Dis. 2021 Sep;15(9):e0009802. DOI: 10.1371/journal.pntd.0009802
[38] Asebe G, Michlmayr D, Mamo G, Abegaz WE, Endale A, Medhin G, Larrick JW, Legesse M. Seroprevalence of Yellow fever, Chikungunya, and Zika virus at a community level in the Gambella Region, South West Ethiopia. PLoS One. 2021;16(7):e0253953. DOI: 10.1371/journal.pone.0253953
[39] Ahmed A, Elduma A, Magboul B, Higazi T, Ali Y. The First Outbreak of Dengue Fever in Greater Darfur, Western Sudan. Trop Med Infect Dis. 2019 Mar;4(1). DOI: 10.3390/tropicalmed4010043
[40] Chen R, Vasilakis N. Dengue--quo tu et quo vadis? Viruses. 2011 Sep;3(9):1562-608. DOI: 10.3390/v3091562
[41] Muñoz-Jordán JL, Collins CS, Vergne E, Santiago GA, Petersen L, Sun W, Linnen JM. Highly sensitive detection of dengue virus nucleic acid in samples from clinically ill patients. J Clin Microbiol. 2009 Apr;47(4):927-31. DOI: 10.1128/JCM.01564-08
[42] Parkash O, Shueb RH. Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques. Viruses. 2015 Oct;7(10):5410-27. DOI: 10.3390/v7102877
[43] Sahak MN. Dengue fever as an emerging disease in Afghanistan: Epidemiology of the first reported cases. Int J Infect Dis. 2020 Oct;99:23-27. DOI: 10.1016/j.ijid.2020.07.033
[44] Ahmed A, Ali Y, Elmagboul B, Mohamed O, Elduma A, Bashab H, Mahamoud A, Khogali H, Elaagip A, Higazi T. Dengue Fever in the Darfur Area, Western Sudan. Emerg Infect Dis. 2019 Nov;25(11):2126. DOI: 10.3201/eid2511.181766
[45] Heydari M, Metanat M, Rouzbeh-Far MA, Tabatabaei SM, Rakhshani M, Sepehri-Rad N, Keshtkar-Jahromi M. Dengue Fever as an Emerging Infection in Southeast Iran. Am J Trop Med Hyg. 2018 May;98(5):1469-71. DOI: 10.4269/ajtmh.17-0634
[46] Nathan MB, Dayal-Drager R, Guzman M. Epidemiology, burden of disease and transmission. WHO dengue guidelines for diagnosis, treatment, prevention and control: New edition. Geneva: World Health Organization; 2009. p. 1-21. Available from: https://www.ncbi.nlm.nih.gov/books/NBK143159/
[47] Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013 Apr;496(7446):504-7. DOI: 10.1038/nature12060
[48] Qamash T, Jamil J, Kalsoom, Khan FA, Saira, Sultan A, Begum N, Din SU. Epidemiological study of dengue fever in District Swabi, Khyber Pakhtunkhwa, Pakistan. Braz J Biol. 2021;81(2):237-40. DOI: 10.1590/1519-6984.216284
[49] Ali A, Fatima Z, Wahid B, Rafique S, Idrees M. Cosmopolitan A1 lineage of dengue virus serotype 2 is circulating in Pakistan: A study from 2017 dengue viral outbreak. J Med Virol. 2019 Nov;91(11):1909-17. DOI: 10.1002/jmv.25537
[50] Desogi M, Ali M, Gindeel N, Khalid F, Abdelraheem M, Alnaby A, Saad M, Elamin E, Kheir M, Mukhtar M. Detection of dengue virus serotype 4 in Sudan. East Mediterr Health J. 2023 Jun;29(6):436-41. DOI: 10.26719/emhj.23.041
[51] Alsedig K, Eldigail MH, Elduma AH, Elaagip A, Altahir O, Siam HA, Ali Y, Abdallah T. Prevalence of malaria and dengue co-infections among febrile patients during dengue transmission season in Kassala, eastern Sudan. PLoS Negl Trop Dis. 2023 Oct;17(10):e0011660. DOI: 10.1371/journal.pntd.0011660
[52] Higa Y. Dengue Vectors and their Spatial Distribution. Trop Med Health. 2011 Dec;39(4 Suppl):17-27. DOI: 10.2149/tmh.2011-S04
[53] Rodrigue Simonet PN, Alexandre Michel NN, Abel W, Albert E, Martin Hermann G, Franziska S. Diversity and Abundance of Potential Vectors of Rift Valley Fever Virus in the North Region of Cameroon. Insects. 2020 Nov;11(11). DOI: 10.3390/insects11110814
[54] Sharp TM, Fischer M, Muñoz-Jordán JL, Paz-Bailey G, Staples JE, Gregory CJ, Waterman SH. Dengue and Zika Virus Diagnostic Testing for Patients with a Clinically Compatible Illness and Risk for Infection with Both Viruses. MMWR Recomm Rep. 2019 Jun;68(1):1-10. DOI: 10.15585/mmwr.rr6801a1
[55] Balkhy HH, Memish ZA. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int J Antimicrob Agents. 2003 Feb;21(2):153-7. DOI: 10.1016/s0924-8579(02)00295-9
[56] Anywaine Z, Lule SA, Hansen C, Warimwe G, Elliott A. Clinical manifestations of Rift Valley fever in humans: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2022 Mar;16(3):e0010233. DOI: 10.1371/journal.pntd.0010233
[57] Smithburn KC, Haddow AJ, Gillett JD. Rift Valley fever; isolation of the virus from wild mosquitoes. Br J Exp Pathol. 1948;29(2):107-21.
[58] Paweska JT, Mortimer E, Leman PA, Swanepoel R. An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in humans, domestic and wild ruminants. J Virol Methods. 2005 Jul;127(1):10-8. DOI: 10.1016/j.jviromet.2005.02.008
[59] Zohaib A, Niazi SK, Saqib M, Sajid MS, Khan I, Sial AU, Athar MA, Taj Z, Abbas G, Rathore MA, Ghani E, Naeem MA, Imran M, Iqbal N, Rehman SU, Waruhiu C, Shi ZL. Detection of West Nile virus lineage 1 sequences in blood donors, Punjab Province, Pakistan. Int J Infect Dis. 2019 Apr;81:137-9. DOI: 10.1016/j.ijid.2019.01.020
[60] Ziyaeyan M, Behzadi MA, Leyva-Grado VH, Azizi K, Pouladfar G, Dorzaban H, Ziyaeyan A, Salek S, Jaber Hashemi A, Jamalidoust M. Widespread circulation of West Nile virus, but not Zika virus in southern Iran. PLoS Negl Trop Dis. 2018 Dec;12(12):e0007022. DOI: 10.1371/journal.pntd.0007022
[61] Niazi AU, Jawad MJ, Amirnajad A, Durr PA, Williams DT. Crimean-Congo Hemorrhagic Fever, Herat Province, Afghanistan, 2017. Emerg Infect Dis. 2019 Aug;25(8):1596-8. DOI: 10.3201/eid2508.181491
[62] Dargham SR, Al-Sadeq DW, Yassine HM, Ahmed M, Kunhipurayil H, Humphrey JM, Abu-Raddad LJ, Nasrallah GK. Seroprevalence of West Nile Virus among Healthy Blood Donors from Different National Populations Residing in Qatar. Int J Infect Dis. 2021 Feb;103:502-6. DOI: 10.1016/j.ijid.2020.11.175
[63] Kemmerly SA. Diagnosis and treatment of west nile infections. Ochsner J. 2003 Summer;5(3):16-7.
[64] Sambri V, Capobianchi MR, Cavrini F, Charrel R, Donoso-Mantke O, Escadafal C, Franco L, Gaibani P, Gould EA, Niedrig M, Papa A, Pierro A, Rossini G, Sanchini A, Tenorio A, Varani S, Vázquez A, Vocale C, Zeller H. Diagnosis of west nile virus human infections: overview and proposal of diagnostic protocols considering the results of external quality assessment studies. Viruses. 2013 Sep;5(10):2329-48. DOI: 10.3390/v5102329
[65] Zakhia R, Dupuis AP 2nd, Khodr F, Fadel M, Kramer LD, Haddad N. Evidence of West Nile Virus Circulation in Lebanon. Viruses. 2021 May;13(6). DOI: 10.3390/v13060994
[66] Obaidat MM, Stringer AP, Roess AA. Seroprevalence, risk factors and spatial distribution of West Nile virus in Jordan. Trans R Soc Trop Med Hyg. 2019 Jan;113(1):24-30. DOI: 10.1093/trstmh/try111
[67] Nasraoui N, Ben Moussa ML, Ayedi Y, Mastouri M, Trabelsi A, Raies A, Wölfel R, Moussa MB. A sero-epidemiological investigation of West Nile virus among patients without any records of their symptoms from three different hospitals from Tunisia. Acta Trop. 2023 Jun;242:106905. DOI: 10.1016/j.actatropica.2023.106905
[68] Khan E, Barr KL, Farooqi JQ, Prakoso D, Abbas A, Khan ZY, Ashi S, Imtiaz K, Aziz Z, Malik F, Lednicky JA, Long MT. Human West Nile Virus Disease Outbreak in Pakistan, 2015-2016. Front Public Health. 2018;6:20. DOI: 10.3389/fpubh.2018.00020
[69] Ahmed A, Ali Y, Elduma A, Eldigail MH, Mhmoud RA, Mohamed NS, Ksiazek TG, Dietrich I, Weaver SC. Unique Outbreak of Rift Valley Fever in Sudan, 2019. Emerg Infect Dis. 2020 Dec;26(12):3030-3. DOI: 10.3201/eid2612.201599
[70] Ahmed SS, Soghaier MA, Mohammed S, Khogali HS, Osman MM, Abdalla AM. Concomitant outbreaks of yellow fever and hepatitis E virus in Darfur States, Sudan, 2012. J Infect Dev Ctries. 2016 Jan;10(1):24-9. DOI: 10.3855/jidc.6342
[71] Adam A, Seidahmed OM, Weber C, Schnierle B, Schmidt-Chanasit J, Reiche S, Jassoy C. Low Seroprevalence Indicates Vulnerability of Eastern and Central Sudan to Infection with Chikungunya Virus. Vector Borne Zoonotic Dis. 2016 Apr;16(4):290-1. DOI: 10.1089/vbz.2015.1897
[72] Domingo C, Charrel RN, Schmidt-Chanasit J, Zeller H, Reusken C. Yellow fever in the diagnostics laboratory. Emerg Microbes Infect. 2018 Jul;7(1):129. DOI: 10.1038/s41426-018-0128-8
[73] Waggoner JJ, Rojas A, Pinsky BA. Yellow Fever Virus: Diagnostics for a Persistent Arboviral Threat. J Clin Microbiol. 2018 Oct;56(10). DOI: 10.1128/JCM.00827-18
[74] de Rezende IM, Oliveira GFG, Costa TA, Khan A, Pereira LS, Santos TA, Alves PA, Calzavara-Silva CE, Martins-Filho OA, Teixeira-Carvalho A, LaBeaud AD, Drumond BP. Yellow Fever Molecular Diagnosis Using Urine Specimens during Acute and Convalescent Phases of the Disease. J Clin Microbiol. 2022 Aug;60(8):e0025422. DOI: 10.1128/jcm.00254-22
[75] Counotte MJ, Kim CR, Wang J, Bernstein K, Deal CD, Broutet NJN, Low N. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med. 2018 Jul;15(7):e1002611. DOI: 10.1371/journal.pmed.1002611
[76] Ahmed SS, Soghaier MA, Mohammed S, Khogali HS, Osman MM, Abdalla AM. Concomitant outbreaks of yellow fever and hepatitis E virus in Darfur States, Sudan, 2012. J Infect Dev Ctries. 2016 Jan;10(1):24-9. DOI: 10.3855/jidc.6342
[77] Asebe G, Michlmayr D, Mamo G, Abegaz WE, Endale A, Medhin G, Larrick JW, Legesse M. Seroprevalence of Yellow fever, Chikungunya, and Zika virus at a community level in the Gambella Region, South West Ethiopia. PLoS One. 2021;16(7):e0253953. DOI: 10.1371/journal.pone.0253953
[78] Ahmed A, Ali Y, Elmagboul B, Mohamed O, Elduma A, Bashab H, Mahamoud A, Khogali H, Elaagip A, Higazi T. Dengue Fever in the Darfur Area, Western Sudan. Emerg Infect Dis. 2019 Nov;25(11):2126. DOI: 10.3201/eid2511.181766
[79] Karkhah A, Nouri HR, Javanian M, Koppolu V, Masrour-Roudsari J, Kazemi S, Ebrahimpour S. Zika virus: epidemiology, clinical aspects, diagnosis, and control of infection. Eur J Clin Microbiol Infect Dis. 2018 Nov;37(11):2035-43. DOI: 10.1007/s10096-018-3354-z
[80] Herrada CA, Kabir MA, Altamirano R, Asghar W. Advances in Diagnostic Methods for Zika Virus Infection. J Med Device. 2018 Dec;12(4):0408021-211. DOI: 10.1115/1.4041086
[81] Voermans JJC, Pas SD, van der Linden A, GeurtsvanKessel C, Koopmans M, van der Eijk A, Reusken CBEM. Whole-Blood Testing for Diagnosis of Acute Zika Virus Infections in Routine Diagnostic Setting. Emerg Infect Dis. 2019 Jul;25(7):1394-6. DOI: 10.3201/eid2507.182000
[82] Cerbino-Neto J, Mesquita EC, Souza TM, Parreira V, Wittlin BB, Durovni B, Lemos MC, Vizzoni A, Bispo de Filippis AM, Sampaio SA, Gonçalves Bde S, Bozza FA. Clinical Manifestations of Zika Virus Infection, Rio de Janeiro, Brazil, 2015. Emerg Infect Dis. 2016 Jul;22(7):1318-20. DOI: 10.3201/eid2207.160375
[83] Soghaier MA, Abdelgadir DM, Abdelkhalig SM, Kafi H, Zarroug IMA, Sall AA, Eldegai MH, Elageb RM, Osman MM, Khogali H. Evidence of pre-existing active Zika virus circulation in Sudan prior to 2012. BMC Res Notes. 2018 Dec;11(1):906. DOI: 10.1186/s13104-018-4027-9
[84] Pielnaa P, Al-Saadawe M, Saro A, Dama MF, Zhou M, Huang Y, Huang J, Xia Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020 Apr;543:34-42. DOI: 10.1016/j.virol.2020.01.015
[85] Yadav PD, Kaur H, Gupta N, Sahay RR, Sapkal GN, Shete AM, Deshpande GR, Mohandas S, Majumdar T, Patil S, Pandit P, Kumar A, Nyayanit DA, Sreelatha KH, Manjusree S, Sami H, Khan HM, Malhotra A, Dhingra K, Gadepalli R, Sudha Rani V, Singh MK, Joshi Y, Dudhmal M, Duggal N, Chabbra M, Dar L, Gawande P, Yemul J, Kalele K, Arjun R, Nagamani K, Borkakoty B, Sahoo G, Praharaj I, Dutta S, Barde P, Jaryal SC, Rawat V. Zika a Vector Borne Disease Detected in Newer States of India Amidst the COVID-19 Pandemic. Front Microbiol. 2022;13:888195. DOI: 10.3389/fmicb.2022.888195
[86] Roth NM, Reynolds MR, Lewis EL, Woodworth KR, Godfred-Cato S, Delaney A, Akosa A, Valencia-Prado M, Lash M, Elmore A, Langlois P, Khuwaja S, Tufa A, Ellis EM, Nestoridi E, Lyu C, Longcore ND, Piccardi M, Lind L, Starr S, Johnson L, Browne SE, Gosciminski M, Velasco PE, Johnson-Clarke F, Locklear A, Chan M, Fornoff J, Toews KE, Tonzel J, Marzec NS, Hale S, Nance AE, Willabus T, Contreras D, Adibhatla SN, Iguchi L, Potts E, Schiffman E, Lolley K, Stricklin B, Ludwig E, Garstang H, Marx M, Ferrell E, Moreno-Gorrin C, Signs K, Romitti P, Leedom V, Martin B, Castrodale L, Cook A, Fredette C, Denson L, Cronquist L, Nahabedian JF 3rd, Shinde N, Polen K, Gilboa SM, Martin SW, Cragan JD, Meaney-Delman D, Honein MA, Tong VT, Moore CA. Zika-Associated Birth Defects Reported in Pregnancies with Laboratory Evidence of Confirmed or Possible Zika Virus Infection - U.S. Zika Pregnancy and Infant Registry, December 1, 2015-March 31, 2018. MMWR Morb Mortal Wkly Rep. 2022 Jan;71(3):73-9. DOI: 10.15585/mmwr.mm7103a1
[87] Lee WL, Gu X, Armas F, Leifels M, Wu F, Chandra F, Chua FJD, Syenina A, Chen H, Cheng D, Ooi EE, Wuertz S, Alm EJ, Thompson J. Monitoring human arboviral diseases through wastewater surveillance: Challenges, progress and future opportunities. Water Res. 2022 Sep;223:118904. DOI: 10.1016/j.watres.2022.118904
[88] Pereira Cabral B, da Graça Derengowski Fonseca M, Mota FB. Long term prevention and vector control of arboviral diseases: What does the future hold? Int J Infect Dis. 2019 Dec;89:169-74. DOI: 10.1016/j.ijid.2019.10.002
[89] Rocklöv J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020 May;21(5):479-83. DOI: 10.1038/s41590-020-0648-y
[90] Obaidat MM, Roess AA. First report on seroprevalence and risk factors of dengue virus in Jordan. Trans R Soc Trop Med Hyg. 2018 Jun;112(6):279-84. DOI: 10.1093/trstmh/try055
[91] Abdalla TM, Karsany MS, Ali AA. Correlation of measles and dengue infection in Kassala, Eastern Sudan. J Med Virol. 2015 Jan;87(1):76-8. DOI: 10.1002/jmv.24001
[92] Zamanian MH, Nouri R, Shirvani M, Mohseniafshar Z, Miladi R, Mehdizad R, et al. Evaluation of Crimean-Congo Hemorrhagic Fever in Kermanshah (2006-2020). Zahedan J Res Med Sci. 2023;25(3):e128662. DOI: 10.5812/zjrms-128662
[93] Umair M, Rehman Z, Haider SA, Ali Q, Hakim R, Bibi S, Salman M, Ikram A. Whole-genome sequencing of Crimean-Congo hemorrhagic fever virus circulating in Pakistan during 2022. J Med Virol. 2023 Mar;95(3):e28604. DOI: 10.1002/jmv.28604
[94] Qureshi H, Khan MI, Bae SJ, Akhtar S, Khattak AA, Haider A, Nisar A. Prevalence of dengue virus in Haripur district, Khyber Pakhtunkhwa, Pakistan. J Infect Public Health. 2023 Jul;16(7):1131-6. DOI: 10.1016/j.jiph.2023.04.021
[95] Marzok M, Alkashif K, Kandeel M, Salem M, Sayed-Ahmed MZ, Selim A. Seroprevalence of Rift Valley Fever virus in one-humped camels (Camelus dromedaries) in Egypt. Trop Anim Health Prod. 2023 Oct;55(5):345. DOI: 10.1007/s11250-023-03765-3
[96] Zohaib A, Saqib M, Athar MA, Hussain MH, Sial AU, Tayyab MH, Batool M, Sadia H, Taj Z, Tahir U, Jakhrani MY, Tayyab J, Kakar MA, Shahid MF, Yaqub T, Zhang J, Wu Q, Deng F, Corman VM, Shen S, Khan I, Shi ZL. Crimean-Congo Hemorrhagic Fever Virus in Humans and Livestock, Pakistan, 2015-2017. Emerg Infect Dis. 2020 Apr;26(4):773-7. DOI: 10.3201/eid2604.191154
[97] Tripathi S, Bhati R, Gopalakrishnan M, Bohra GK, Tiwari S, Panda S, Sahay RR, Yadav PD, Nag VL, Garg MK. Clinical profile and outcome of patients with Crimean Congo haemorrhagic fever: a hospital based observational study from Rajasthan, India. Trans R Soc Trop Med Hyg. 2020 Sep;114(9):643-9. DOI: 10.1093/trstmh/traa014
[98] Shahid MF, Shabbir MZ, Ashraf K, Ali M, Yaqub S, Ul-Rahman A, Sardar N, Mukhtar N, Tahir Z, Yaqub T. Sero-Epidemiological Survey of Crimean-Congo Hemorrhagic Fever among the Human Population of the Punjab Province in Pakistan. Virol Sin. 2020 Aug;35(4):486-9. DOI: 10.1007/s12250-020-00195-5
[99] Al-Abri SS, Hewson R, Al-Kindi H, Al-Abaidani I, Al-Jardani A, Al-Maani A, Almahrouqi S, Atkinson B, Al-Wahaibi A, Al-Rawahi B, Bawikar S, Beeching NJ. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in Oman. PLoS Negl Trop Dis. 2019 Apr;13(4):e0007100. DOI: 10.1371/journal.pntd.0007100
[100] Aslani D, Salehi-Vaziri M, Baniasadi V, Jalali T, Azad-Manjiri S, Mohammadi T, Khakifirouz S, Fazlalipour M. Crimean-Congo hemorrhagic fever among children in Iran. Arch Virol. 2017 Mar;162(3):721-5. DOI: 10.1007/s00705-016-3162-7
[101] Karlberg H, Sharifi-Mood B, Mousavi-Jazi M, Dilcher M, Lindegren G, Mardani M, Bereskly S, Weidmann M, Mirazimi A. Molecular and serological findings in suspected patients with Crimean-Congo hemorrhagic fever virus in Iran. J Med Virol. 2015 Apr;87(4):686-93. DOI: 10.1002/jmv.24106
[102] Baudin M, Jumaa AM, Jomma HJE, Karsany MS, Bucht G, Näslund J, Ahlm C, Evander M, Mohamed N. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health. 2016 Nov;4(11):e864-e871. DOI: 10.1016/S2214-109X(16)30176-0
[103] Hussen MO, Sayed ASM, Abushahba MFN. Sero-epidemiological study on Dengue fever virus in humans and camels at Upper Egypt. Vet World. 2020 Dec;13(12):2618-24. DOI: 10.14202/vetworld.2020.2618-2624
[104] Iqbal G, Javed H, Raza FA, Gohar UF, Fatima W, Khurshid M. Diagnosis of Acute Dengue Virus Infection Using Enzyme-Linked Immunosorbent Assay and Real-Time PCR. Can J Infect Dis Med Microbiol. 2023;2023:3995366. DOI: 10.1155/2023/3995366
[105] Swedan S, Al-Saleh D. Transfusion transmitted virus and dengue virus among healthy blood donors: A prevalence report from Jordan. Biomol Biomed. 2023;23(3):450-6. DOI: 10.17305/bjbms.2022.7832
[106] Al Balushi L, Al Kalbani M, Al Manji A, Amin M, Al Balushi Z, Al Barwani N, Al Wahaibi A, Al Manji A, Al Kindi H, Petersen E, Al Ghafri T, Al-Abri S. A second local dengue fever outbreak: A field experience from Muscat Governorate in Oman, 2022. IJID Reg. 2023 Jun;7:237-41. DOI: 10.1016/j.ijregi.2023.03.015
[107] El-Kady AM, Osman HA, Alemam MF, Marghani D, Shanawaz MA, Wakid MH, Al-Megrin WAI, Elshabrawy HA, Abdella OH, Allemailem KS, Almatroudi A, El-Amir MI. Circulation of Dengue Virus Serotype 2 in Humans and Mosquitoes During an Outbreak in El Quseir City, Egypt. Infect Drug Resist. 2022;15:2713-21. DOI: 10.2147/IDR.S360675
[108] Mukhtar MU, Mukhtar M, Iqbal N, Nawaz Z, Bhatti A, Haq F, Arslan A, Rashid M. The emergence of Dengue Fever in Sheikhupura, Pakistan: its seroprevalence and risk factors assessment during 2014-2017. J Infect Dev Ctries. 2021 Sep;15(9):1351-5. DOI: 10.3855/jidc.13976
[109] Ahmed A, Eldigail M, Elduma A, Breima T, Dietrich I, Ali Y, Weaver SC. First report of epidemic dengue fever and malaria co-infections among internally displaced persons in humanitarian camps of North Darfur, Sudan. Int J Infect Dis. 2021 Jul;108:513-6. DOI: 10.1016/j.ijid.2021.05.052
[110] Eldigail MH, Abubaker HA, Khalid FA, Abdallah TM, Musa HH, Ahmed ME, Adam GK, Elbashir MI, Aradaib IE. Association of genotype III of dengue virus serotype 3 with disease outbreak in Eastern Sudan, 2019. Virol J. 2020 Jul;17(1):118. DOI: 10.1186/s12985-020-01389-9
[111] Elaagip A, Alsedig K, Altahir O, Ageep T, Ahmed A, Siam HA, Samy AM, Mohamed W, Khalid F, Gumaa S, Mboera L, Sindato C, Elton L, Zumla A, Haider N, Kock R, Abdel Hamid MM. Seroprevalence and associated risk factors of Dengue fever in Kassala state, eastern Sudan. PLoS Negl Trop Dis. 2020 Dec;14(12):e0008918. DOI: 10.1371/journal.pntd.0008918
[112] Eldigail MH, Abubaker HA, Khalid FA, Abdallah TM, Adam IA, Adam GK, Babiker RA, Ahmed ME, Haroun EM, Aradaib IE. Recent transmission of dengue virus and associated risk Facors among residents of Kassala state, eastern Sudan. BMC Public Health. 2020 Apr;20(1):530. DOI: 10.1186/s12889-020-08656-y
[113] Khan NU, Danish L, Khan HU, Shah M, Ismail M, Ali I, Petruzziello A, Sabatino R, Guzzo A, Botti G, Iqbal A. Prevalence of dengue virus serotypes in the 2017 outbreak in Peshawar, KP, Pakistan. J Clin Lab Anal. 2020 Sep;34(9):e23371. DOI: 10.1002/jcla.23371
[114] Hamid Z, Hamid T, Alsedig K, Abdallah T, Elaagip A, Ahmed A, Khalid F, Abdel Hamid M. Molecular Investigation of Dengue Virus Serotype 2 Circulation in Kassala State, Sudan. Jpn J Infect Dis. 2019 Jan;72(1):58-61. DOI: 10.7883/yoken.JJID.2018.267
[115] Mukhtar MU, Mukhtar M, Iqbal N. Dengue fever is an emerging public health concern in the city of Multan, Pakistan: its seroprevalence and associated risk factors. Microbiol Immunol. 2018 Nov;62(11):729-31. DOI: 10.1111/1348-0421.12649
[116] Khan J, Ghaffar A, Khan SA. The changing epidemiological pattern of Dengue in Swat, Khyber Pakhtunkhwa. PLoS One. 2018;13(4):e0195706. DOI: 10.1371/journal.pone.0195706
[117] Raza FA, Ashraf S, Hasnain S, Ahmad J, Iqbal M. Dengue seroprevalence and its socioeconomic determinants in Faisalabad, Pakistan: a cross-sectional study. Rev Soc Bras Med Trop. 2018;51(4):503-7. DOI: 10.1590/0037-8682-0246-2017
[118] Adam A, Schüttoff T, Reiche S, Jassoy C. High seroprevalence of dengue virus indicates that dengue virus infections are frequent in central and eastern Sudan. Trop Med Int Health. 2018 Sep;23(9):960-7. DOI: 10.1111/tmi.13116
[119] Suleman M, Faryal R, Alam MM, Sharif S, Shaukat S, Aamir UB, Khurshid A, Angez M, Umair M, Sufian MM, Arshad Y, Zaidi SS. Dengue Virus Serotypes Circulating in Khyber Pakhtunkhwa Province, Pakistan, 2013-2015. Ann Lab Med. 2017 Mar;37(2):151-4. DOI: 10.3343/alm.2017.37.2.151
[120] Suleman M, Lee HW, Zaidi SS, Alam MM, Nisar N, Aamir UB, Sharif S, Shaukat S, Khurshid A, Angez M, Umair M, Mujtaba G, Faryal R. Preliminary Seroepidemiological survey of dengue infections in Pakistan, 2009-2014. Infect Dis Poverty. 2017 Mar;6(1):48. DOI: 10.1186/s40249-017-0258-6
[121] Suleman M, Faryal R, Alam MM, Khurshid A, Sharif S, Shaukat S, Angez M, Umair M, Sufian MM, Arshad Y, Inam T, Zaidi SSZ. Outbreak of dengue virus type-3 in Malakand, Pakistan 2015; A laboratory perspective. Acta Trop. 2017 May;169:202-6. DOI: 10.1016/j.actatropica.2017.02.011
[122] Ahmed I, Reza FA, Iqbal M, Ashraf M. Dengue virus serotypes and epidemiological features of dengue fever in Faisalabad, Pakistan. Trop Biomed. 2017 Dec 1;34(4):928-35.
[123] Ali A, Ahmad H, Idrees M, Zahir F, Ali I. Circulating serotypes of dengue virus and their incursion into non-endemic areas of Pakistan; a serious threat. Virol J. 2016;13(1):144. DOI: 10.1186/s12985-016-0603-6
[124] Khan E, Farooqi JQ, Barr KL, Prakoso D, Nasir A, Kanji A, et al. Flaviviruses as a cause of undifferentiated fever in Sindh Province, Pakistan: A preliminary report. Front Public Health. 2016;4:8. doi: 10.3389/fpubh.2016.00008
[125] Elyan DS, Moustafa L, Noormal B, Jacobs JS, Aziz MA, Hassan KS, Wasfy MO, Monestersky JH, Oyofo BA. Serological evidence of flaviviruses infection among acute febrile illness patients in Afghanistan. J Infect Dev Ctries. 2014 Sep;8(9):1176-80. DOI: 10.3855/jidc.4183
[126] Wilson ML, Chapman LE, Hall DB, Dykstra EA, Ba K, Zeller HG, Traore-Lamizana M, Hervy JP, Linthicum KJ, Peters CJ. Rift Valley fever in rural northern Senegal: human risk factors and potential vectors. Am J Trop Med Hyg. 1994 Jun;50(6):663-75. DOI: 10.4269/ajtmh.1994.50.663
[127] Ndumu DB, Bakamutumaho B, Miller E, Nakayima J, Downing R, Balinandi S, Monje F, Tumusiime D, Nanfuka M, Meunier N, Arinaitwe E, Rutebarika C, Kidega E, Kyondo J, Ademun R, Njenga KM, Veas F, Gonzalez JP. Serological evidence of Rift Valley fever virus infection among domestic ruminant herds in Uganda. BMC Vet Res. 2021 Apr;17(1):157. DOI: 10.1186/s12917-021-02867-0
[128] Doyle MP, Genualdi JR, Bailey AL, Kose N, Gainza C, Rodriguez J, Reeder KM, Nelson CA, Jethva PN, Sutton RE, Bombardi RG, Gross ML, Julander JG, Fremont DH, Diamond MS, Crowe JE Jr. Isolation of a Potently Neutralizing and Protective Human Monoclonal Antibody Targeting Yellow Fever Virus. mBio. 2022 Jun;13(3):e0051222. DOI: 10.1128/mbio.00512-22
[129] Aziz A, Suleman M, Shah A, Ullah A, Rashid F, Khan S, Iqbal A, Luo S, Xie L, Xie Z. Comparative mutational analysis of the Zika virus genome from different geographical locations and its effect on the efficacy of Zika virus-specific neutralizing antibodies. Front Microbiol. 2023;14:1098323. DOI: 10.3389/fmicb.2023.1098323




