[Wiederbelebung der Cephalosporine: Das Potential von β-Lactamase-Hemmer Kombinationen]
Kazi-Chishti Marzooka 1Shaikh Sajed 1
Mohamed Hassan Dehghan 1
Kazi Bilal 2
Rumani Imaan 3
1 Department of Pharmaceutics, Maulana Azad Educational Trust’s, Y. B. Chavan College of Pharmacy, Dr. Rafiq Zakaria Campus, Aurangabad, Maharashtra, India
2 Department of Hematology, Bone Marrow Transplantation and Cellular Therapy, SunAct Cancer Institute, Mumbai, India
3 Department of Surgical Oncology, SunAct Cancer Institute, Mumbai, India
Zusammenfassung
Die Übersicht befasst sich mit der Zunahme multiresistenter (MDR) Gram-negativer Bakterien, wobei β-Lactamase-Inhibitor-Kombinationen als wichtige therapeutische Optionen hervorgehoben werden.
Untersucht werden β-Lactam-Resistenzmechanismen, etablierte Kombinationen (z.B. Ticarcillin/Clavulansäure, Piperacillin/Tazobactam) und die klinische Wirksamkeit neuerer Therapien wie Ceftazidim/Avibactam (CAZ-AVI) gegen Carbapenem-resistente Klebsiella pneumoniae (CRKP) und Ceftolozan/Tazobactam (TOL-TAZ) gegen MDR-Pseudomonas aeruginosa. Darüber hinaus werden neuartige Kombinationen (z.B. Cefepim-Enmetazobactam, Cefepim-Taniborbactam) zur Bekämpfung extensiv arzneimittelresistenter (XDR) Bakterien diskutiert.
Durch vergleichende Analysen liefert die Übersicht wichtige Erkenntnisse über Wirksamkeit, Resistenz, Pharmakokinetik und Sicherheit, die bei der Optimierung antimikrobieller Strategien und Klinikern bei der Behandlung von MDR-Infektionen helfen und gleichzeitig das Antibiotikamanagement und die zukünftige Forschung unterstützen.
Schlüsselwörter
Beta-Lactamase-Hemmer Kombinationen, Gram-negative Bakterien, Antibiotikaresiste
Introduction
β-Lactams are the most widely used antibiotics globally, and include various penicillin derivatives and related classes such as cephalosporins, cephamycins, carbapenems, monobactams, and penems [1], [2]. Despite their effectiveness, bacteria have developed resistance mechanisms, including the production of β-lactamase enzymes that break down the antibiotic structure. β-Lactamases are classified into four categories: A, B, C, and D, based on their structure and hydrolytic mechanisms. Class B enzymes, called metallo-β-lactamases (MBLs), require zinc ions for activity, while classes A, C, and D use serine [2], [3]. The overuse of β-lactams has contributed to the growing resistance to extended-spectrum cephalosporins and carbapenems, posing a serious global health threat. Particularly concerning are carbapenem-resistant bacteria, such as those encoding Klebsiella (K.) pneumoniae carbapenemase (KPC), New Delhi metallo-β-lactamase (NDM), and oxacillinase (OXA-48), due to their ability to resist multiple antibiotics. These growing resistances underscore the urgent need for effective antimicrobial strategies to address resistant bacterial infections and protect public health. A promising approach is the development of broad-spectrum β-lactamase inhibitors, designed to counteract β-lactam resistance by blocking the enzymes responsible for breaking down antibiotics like cephalosporins and carbapenems. These inhibitors target common pathogens treated with β-lactams, including Escherichia (E.) coli, K. pneumoniae, and Pseudomonas (P.) aeruginosa [1], [2], [3]. Resistance to β-lactams arises through various mechanisms, such as changes in membrane permeability, enzyme inactivation, and efflux pumps. Horizontal gene transfer, especially among carbapenemases-producing bacteria, plays a major role in spreading resistance [4], [5].This work aims to provide a detailed review of current β-lactamase inhibitors and their combinations, as well as explore newly developed or experimental compounds.
Mechanism of action of β-lactam antibiotics and antibiotic resistance
β-Lactam antibiotics disrupt bacterial cell wall synthesis by mimicking the D-alanine-D-alanine structure in peptidoglycans, thereby inhibiting isopeptide bond formation by bacterial transpeptidases [6]. This interference reduces bacterial growth and division, while also weakening their defense against osmotic or tensile stress [7]. Penems, like faropenem, target L,D-transpeptidases, unlike carbapenems that focus on D,D-transpeptidases, making penems uniquely effective against mycobacteria [8], [9]. Penicillin-binding proteins (PBPs), essential for peptide cross-linking, are key targets of β-lactam antibiotics. PBPs are classified by molecular mass into various classes and subclasses [10], [11], [12], [13]. In Gram-negative bacteria, high-molecular-weight PBPs such as 1a, 1b, PBP2, and PBP3 play critical roles, and their inhibition can lead to cell death [14], [15].
For decades, β-lactam antibiotics have revolutionized the treatment of bacterial infections. However, their effectiveness is increasingly compromised by the rise and spread of resistance mechanisms, especially in Gram-negative bacteria. These mechanisms (Figure 1 [Fig. 1]) include the production of β-lactamase enzymes, such as extended-spectrum β-lactamases (ESBLs), which inactivate β-lactams and severely limit treatment options [16], [17]. Additionally, mutations in penicillin-binding proteins (PBPs) reduce β-lactams’ binding affinity, diminishing their effectiveness, making it crucial to identify specific PBP mutations for optimized treatment [18], [19]. Efflux pumps, membrane proteins that expel β-lactams from bacterial cells, also play a role in reducing drug efficacy and fostering resistance [20]. Furthermore, changes in the bacterial outer membrane, including altered porins and modifications to the lipopolysaccharide (LPS) layer, prevent β-lactams from entering the cell, lowering of periplasmic accumulation and therapeutic effectiveness [21], [22].
Figure 1: Resistance mechanism of the β-lactam antibiotic (created with BioRender.com)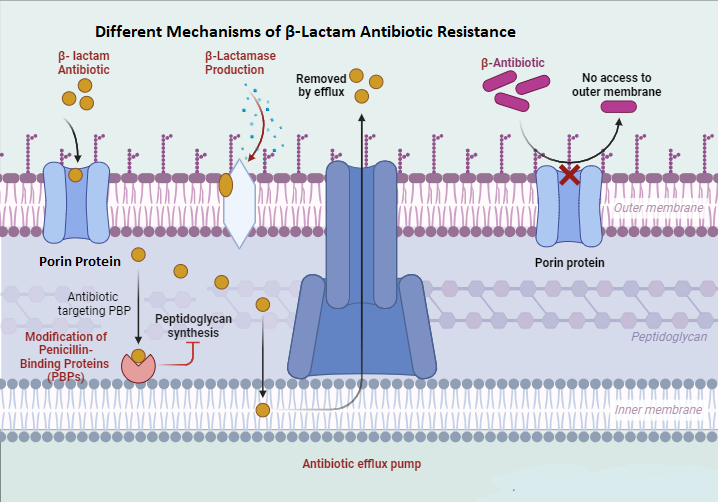
β-Lactamase classification systems
The two primary classification systems for β-lactamases are the Ambler molecular classification and the Bush–Jacoby–Medeiros functional classification. These systems provide complementary information for understanding the enzymes that can inactivate β-lactam antibiotics [23], [24], [25].These classification are described in Table 1 [Tab. 1].
Table 1: Classification of β-lactamases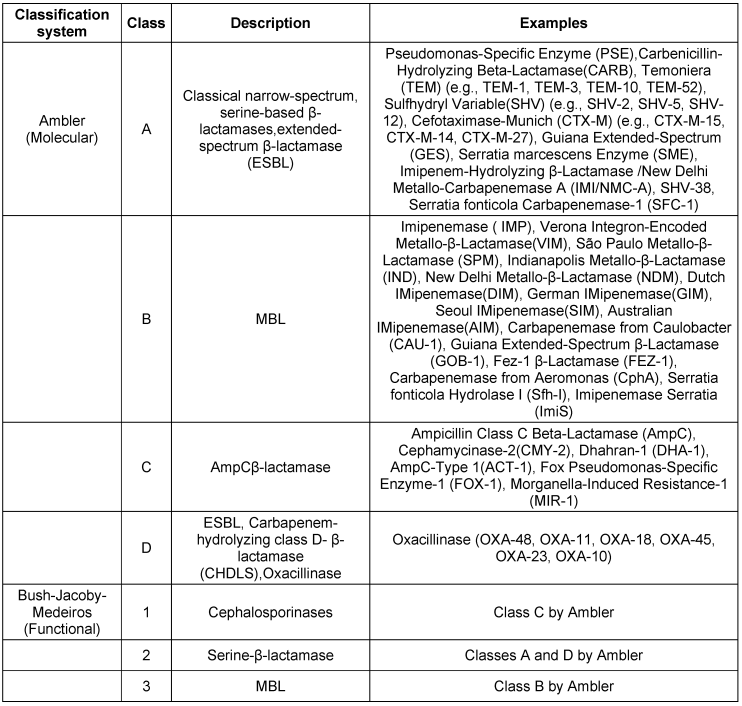
β-Lactamase diversity in Gram-negative bacteria
The increasing diversity of β-lactamase genes in Gram-negative bacteria demandsthe continuous monitoring and development of new strategies to combat antibiotic resistance. Understanding these enzymes and their impact is key to preserving β-lactam effectiveness. Table 2 [Tab. 2] provides an overview of β-lactamase diversity in Gram-negative bacteria and strategies for combating antibiotic resistance for clinically relevant β-lactamase types and their activity spectrum.
Table 2: Clinically relevant β-lactamase types and their activity spectrum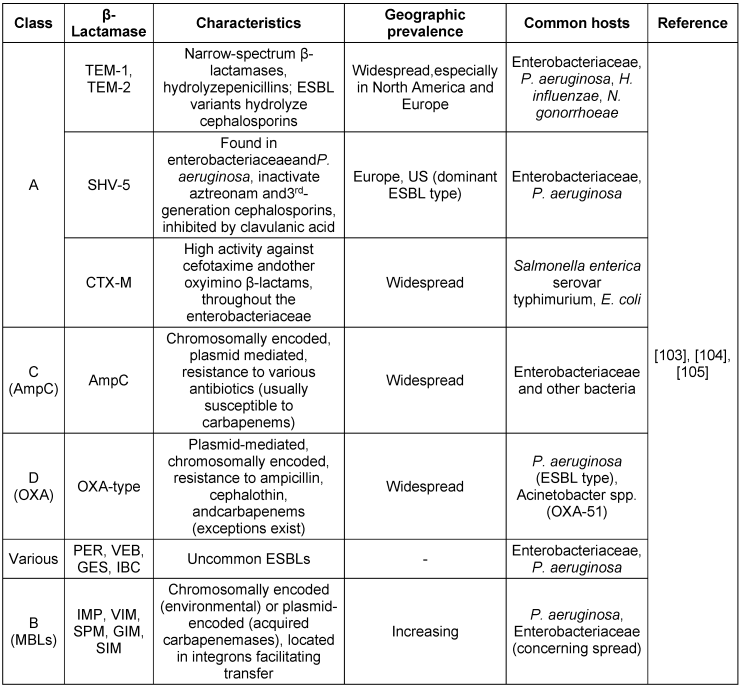
β-Lactam/β-lactamase inhibitor combinations in combating bacterial resistance
The following combinations are described: Ceftazidime-avibactam, ceftalozone-tazobactam, cefepime-zidebactam, cefepime-enmetazobactam and cefepime-taniborbactam.
1. Ceftazidime–avibactam(CAZ-AVI)
The misuse of antibiotics is fuelling a global health crisis, particularly concerning antimicrobial resistance (AMR), especially with CRKP [26], [27]. Carbapenems, once the last resort, are losing effectiveness against CRKP, leaving limited treatment options [26], [27]. This issue is especially alarming in countries such as China [28]. Urgent action is needed for new drugs to combat CRKP, as current strategies such as double-carbapenem therapy have limitations [29], [30]. Fortunately, a new generation of antibiotics, including plazomicin, eravacycline, meropenem-vaborbactam, and CAZ-AVI, is promising [31], [32]. The FDA approval of CAZ-AVI in February 2015 marked a milestone in the battle against challenging bacterial infections. This novel medication, combining a beta-lactamase inhibitor with a cephalosporin, offers a promising treatment option for complicated urinary tract and intra-abdominal infections. Its formulation addresses resistance mechanisms, offering new hope for effective treatment where traditional antibiotics have faltered. These medications, such as AVYCAZ® (Allergan) and ZAVICEFTA® (Pfizer) [33], have since become therapeutic options in the United States and are authorized for a range of serious infections caused by specific susceptible Gram-negative microorganisms in adults aged ≥18 years. Since its introduction on the Chinese market in September 2019, CAZ-AVI has garnered considerable attention for its proven clinical efficacy against carbapenem-resistant CRKPinfections. By inhibiting enzymes such asAmpC-producing beta-lactamase, ESBL, KPC, and OXA-48 carbapenemase, CAZ-AVI has emerged as a crucial weapon in China’s fight against CRKP, demonstrating its value as a vital addition to the global antimicrobial arsenal [34], [35].
Mode of action
Third-generation cephalosporins such as ceftazidime function similarly to other beta-lactam antibiotics. It works by attaching itself to PBPs and preventing bacterial cell wall peptidoglycan synthesis from occurring. Because of this disruption, proper cross-linking during the formation of the cell wall is prevented, which ultimately causes bacterial cell lysis and death [36], [37]. One of the first non-beta-lactam beta-lactamase inhibitors is avibactam. Despite having no inherent antibacterial activity, ceftazidime-avibactam plays a vital role in preventing ceftazidime from being broken down by different serine beta-lactamases [38], [39], [40]. Avibactam protects against ceftazidime via a gradual, reversible process of covalent acylation of beta-lactamase targets, which ultimately releases intact avibactam without hydrolysis. With the exception ofits activity against class B enzymes (MBL), its range of action includes Ambler class A (TEM-1, CTX-M-15, KPC-2, KPC-3), class C (AmpC), and certain class D beta-lactamases (OXA-48) [41], [42], [43].
Bacterial susceptibility and resistance profile
Different beta-lactamases, including class A ESBLs, class B carbapenemases, and class C cephalosporinases, can hydrolyse CAZ. Inhibiting class A, class C, and some class D beta-lactamases, such as CAZ and AVI, provides broad protection against Gram-negative bacteria. However, its effectiveness against Gram-positive bacteria, Gram-negative anaerobes, and isolates that produce class B beta-lactamases is limited [44], [45]. Clinical data show that CAZ-AVI is effective against isolates of enterobacterales that produce AmpC and ESBLs, among other beta-lactamase-producing bacteria. However, it has limited or no activity against OXA-24, OXA-40, and OXA-69 in Acinetobacter (A.) baumannii, and limited activity against OXA-48 in K. pneumoniae, which are specific class D carbapenemases. Global surveillance INFORM (International Network for Optimal Resistance Monitoring) studies revealed high susceptibility rates (99.5% to 100%) of enterobacteriaceae to CAZ-AVI, including isolates of K. pneumoniae, Proteus (P.) mirabilis, E. coli, and K. oxytoca that produce AmpC and ESBL [46].
Recent studies indicate that the combination of CAZ-AVI and aztreonam effectively combats resistant enterobacterisolates carrying the blaNDM-1 and blaKPC-4 genes. Avibactam’s resistance to various enzyme classes prevents NDM from hydrolyzing aztreonam, enhancing their synergistic effect. Additionally, this combination has successfully treated persistent bacteremia caused by Stenotrophomonas maltophilia with L1 (MBL) and L2 (cephalosporinase) beta-lactamases [47].While CAZ-AVI has been an effective option, the newly approved EMBLAVEO® (aztreonam-avibactam) offers significant advantages. Unlike previous combinations, EMBLAVEO restores aztreonam’s activity against bacteria that co-produce MBLs and other β-lactamases more effectively, making it a superior choice for multidrug-resistant Gram-negative infections. The resistance profile of CAZ-AVI is outlined in Table 3 [Tab. 3]. With this new formulation, clinicians can expect improved treatment outcomes and broader efficacy in tackling resistant infections.
Table 3: Resistance profile of ceftazidime-avibactam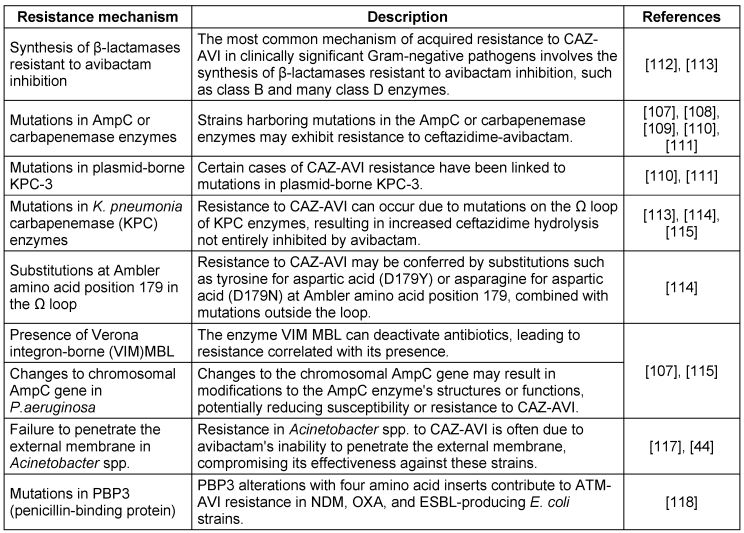
Pharmacokinetic-pharmacodynamic (PK-PD) profile
CAZ and AVI exhibit comparable pharmacokinetic characteristics, such as short plasma half-lives, minimal binding to plasma proteins, and equivalent distribution within the epithelial lining fluid (ELF) [48], [49]. For both medications, the main route of elimination is renal excretion, as described in Table 4 [Tab. 4], while the key PK-PD profile is listed in Table 5 [Tab. 5].
Table 4: Pharmacokinetic characteristics of ceftazidime-avibactam [48], [49]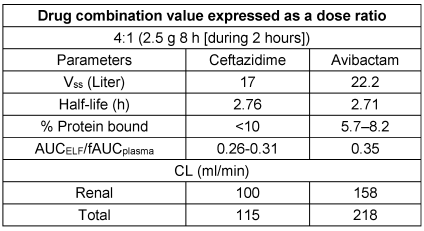
Table 5: Ceftazidime-avibactam: Key aspects of PK-PD
The efficacy of CAZ-AVI in diverse patient populations and bacterial species is detailed in Table 6 [Tab. 6].
Table 6: Ceftazidime-avibactam treatment outcomes in diverse patient populations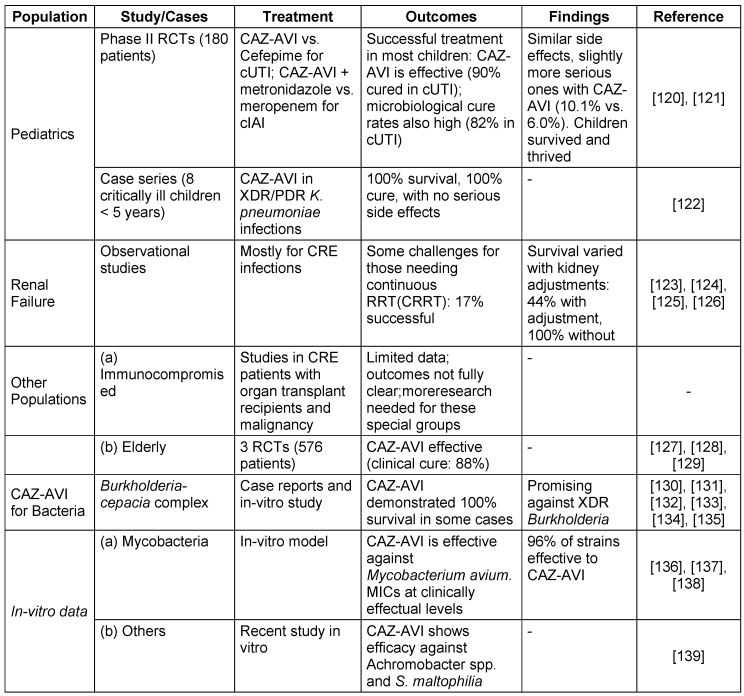
Safety profile and adverse events associated with CAZ-AVI usage
Compared with randomized controlled trials (RCTs), CAZ-AVI did not significantly differ in overall adverse event rates. However, CAZ-AVI was associated with a higher frequency of specific adverse events, including gastrointestinal issues (more than 20% of patients) and mild creatinine level elevations (≤2%). Additionally, 3% to 6% of patients experienced neurological adverse events, pyrexia, peripheral edema, hypersensitivity reactions, and other adverse events. Higher rates of serious adverse events (SAEs) were reported with CAZ-AVI, although detailed descriptions were lacking in the trials [50]. Nonrandomized studies have indicated that up to 5% of patients receiving CAZ-AVI experience acute kidney injury (AKI). Neurological and gastrointestinal side effects are also common, along with infrequent instances of leukopenia, rash, and abnormal liver function. However, these studies lacked thorough reporting of adverse events
2. Ceftalozone–tazobactam (TOL-TAZ)
The FDA and European Medicines Agency have approved TOL-TAZ, a novel combination of a β-lactamase inhibitor and a fifth-generation cephalosporin, for the treatment of several difficult adult infections. Officially approved for the treatment of complicated intra-abdominal infections, complicated urinary tract infections, pyelonephritis, and HABP, it has also demonstrated efficacy in the management of acute pulmonary cystic fibrosis exacerbations in adults; however, formal approval for this particular use has not been granted [51], [52].
Mode of action
Ceftolozane, a member of the cephalosporin class, shares structural similarities with ceftazidime. However, it differs in having a modified pyrazole side chain at the 3-position, which enhances its effectiveness against P. aeruginosa. Its mode of action involves targeting PBPs to inhibit bacterial cell-wall synthesis. Compared with ceftazidime, ceftolozane has a greater affinity for P. aeruginosa PBPs 1b, 1c, and 3 and shows greater stability against AmpC β-lactamase-mediated resistance, which is commonly observed in P. aeruginosa strains. Tazobactam, the β-lactamase inhibitor in this combination, irreversibly inhibits most class A and some class C β-lactamases. This extends the spectrum of ceftolozane activity to include most ESBL-producing Gram-negative bacteria and provides some activity against anaerobic organisms. The fixed-dose combination of ceftolozane and tazobactam is available at a 2:1 ratio, which has been determined to be the most potent combination through studies comparing various ratios (2:1, 4:1, and 8:1) [53].
Bacterial susceptibility and resistance profile
The antipseudomonal combination of TOL-TAZ is effective against a broad range of common Gram-negative pathogens. It has shown efficacy against various Streptococcus species, multidrug-resistant P. aeruginosa, ESBL-producing enterobacterales such as E. coli with CTX-M-14 and CTX-M-15, and some anaerobes such as Bacteroides fragilis and other non-Bacteroides Gram-negative bacteria [54], [55]. Ceftolozane is primarily responsible for the antibacterial effect against P. aeruginosa, with tazobactam contributing minimally. Tazobactam’s main function is to enhance ceftolozane’s activity against Enterobacteriaceae, although the combination shows relatively weak efficacy against E. cloacae. Bacterial susceptibility and resistance profiles are detailed in Table 7 [Tab. 7] and Table 8 [Tab. 8].
Table 7: Ceftolozane-Tazobactam susceptibility in key bacterial populations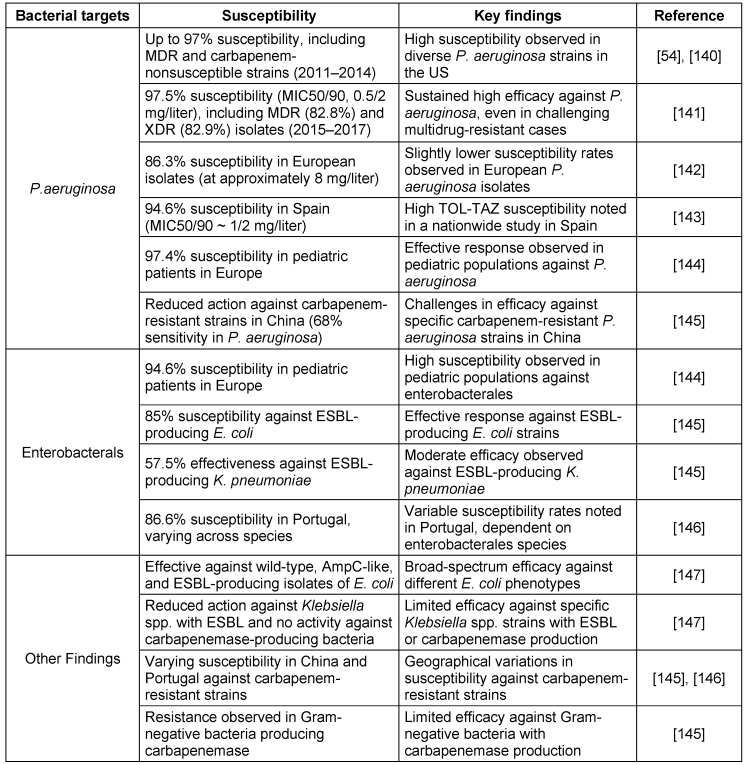
Table 8: Resistance profile of Ceftolozane-Tazobactam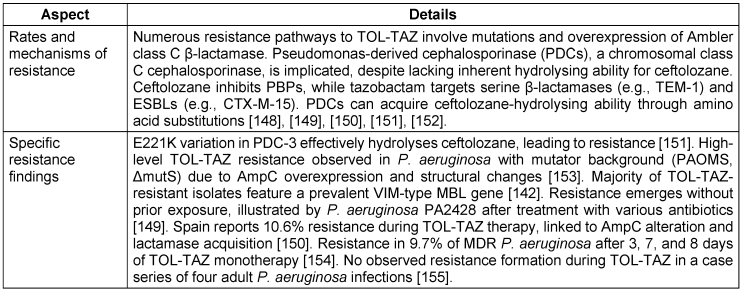
Pharmacokinetic-therapy and pharmacodynamic profile
The volume of distribution at the steady state (Vss) for TOL is 13.5 liters, whereas TAZ has a Vss of 18.2 liters. TOL has a longer half-life (3.12 hours) than TAZ (1.03 hours). TOL is 16–21% protein bound, and TAZ is 30% protein bound. TOL has an AUCELF/fAUC plasma ratio of 0.50, whereas TAZ has a ratio of 0.62. TOL has a renal clearance range of 57–112 ml/min, and TAZ has a renal clearance rate of 210 ml/min. TOL has a total clearance range of 68–112 ml/min, and TAZ has a total clearance of 340 ml/min. Compared with TAZ, TOL results in a lower Vss, longer half-life, lower percentage of protein binding, and distinct clearance patterns [56], [57], the key aspects of PK-PD are highlighted in Table 9 [Tab. 9]. Thus, in combination therapy, the distinct pharmacokinetic profiles of TOL and TAZ offer complementary advantages, potentially enhancing the overall effectiveness of treatment.
Table 9: Key aspects of pharmacokinetic therapy and pharmacodynamic profile of Ceftolozane and tazobactam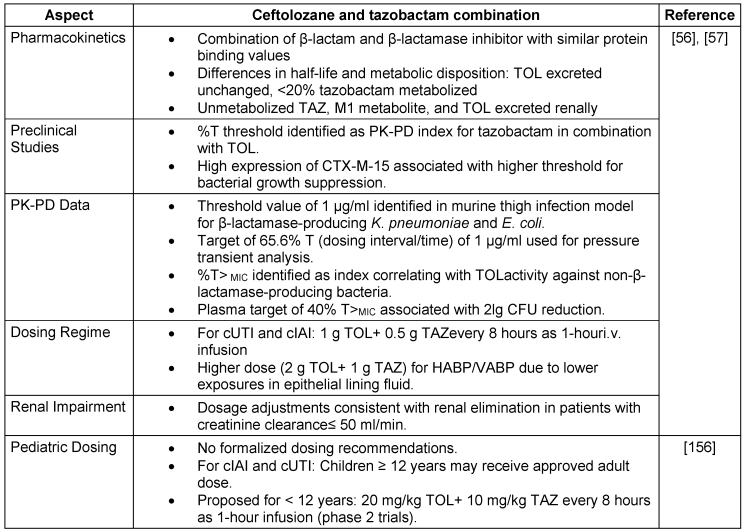
The TOL-TAZ efficacy in diverse patient populations and bacterial species is detailed in Table 10 [Tab. 10].
Table 10: Ceftolozane and tazobactam treatment outcomes in diverse patient populations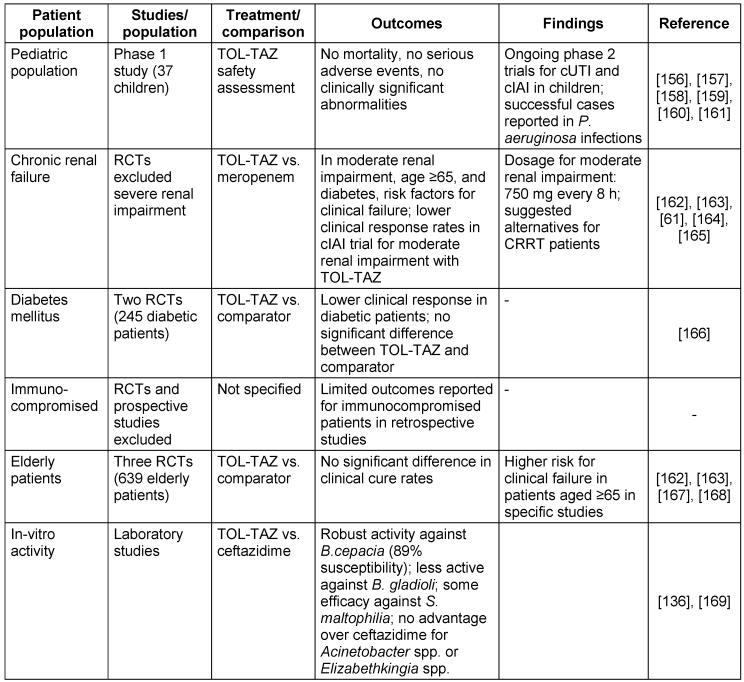
Safety profile and adverse events
The safety profile of TOL-TAZ was generally comparable to that of the comparator in clinical trials. Common side effects included gastrointestinal issues, C. difficile infections, headaches, pyrexia, and abnormal liver function tests. Interestingly, the results of the trial in which high-dose TOL-TAZ was used for pneumonia suggested a greater risk of serious adverse events. In trials with a standard TOL-TAZ dose of 1.5 g every 8 hours, approximately 58% to 62% of the patients reported any adverse events, with 17.5% to 19% being drugrelated. Noteworthy side effects in these cases were gastrointestinal symptoms, sleeplessness, and abnormal liver function tests [58], [59]. In a study by Pogue et al. [60], 63% of 100 patients with MDR/XDR P. aeruginosa infections were on high-dose TOL-TAZ. Among the clinical outcomes, six cases of acute renal damage and four instances of C. difficile infection were noted, although safety data were not separately discussed. In a study conducted by Bassetti et al. [61], [62], drug-related adverse events were observed in 101 patients. Among the reported adverse events, gastrointestinal issues and abnormal liver function test results were identified as the primary concerns. Approximately 30% of these patients received high-dose TOL-TAZ.
3. Cefepime–zidebactam (CEP-ZID)
CEP and ZID (WCK 5222®) are unique combinations; zidebactam is a bicycloacyl hydrazide component that has built-in antibacterial action in addition to acting as a β-lactamase inhibitor. Zidebactam’s dual action protects cefepime against β-lactamase hydrolysis and simultaneously expands its antibacterial range. The in-vitro effectiveness of CEP–ZID and other antibacterial agents on a panel of clinical isolates that were well characterized and resistant to several drugs was recently examined. This investigation focused especially on a variety of carbapenemase manufacturers, resulting in a thorough evaluation of the combination’s efficacy against challenging Gram-negative isolates. The studyelucidated the efficacy of CEP-ZID in light of changing resistance patterns and new threats from MDR bacteria [63].
Although ZID is a non-β-lactam compound with inhibitory effects on class A and MBLs and targets PBP2, it still belongs to the diazabicyclooctane (DBO) class, much like avibactam. Unlike the four recently approved β-lactam/β-lactamase inhibitor combinations, ZID uniquely impacts MBLs. While CEP-ZID has shown promising in-vitro activity against MBL-positive pathogens, it is not yet approved for clinical use. Recent studies have shown a notable 90% to 100% susceptibility to CEP-ZID among 35 MBL-positive CPE strains, including those co-producing serine β-lactamases [64]. The rise of MDR Gram-negative bacteria is a major threat. CEP kills bacteria by disabling cell-wall formation, whereas ZID protects CEP from breakdown and potentially aids in cell-wall disruption. This synergy broadens the effectiveness of CEP-ZID against resistant bacteria, including those with MBL enzymes, which is a growing concern. Early studies revealed promising activity against MDR bacteria, particularly MBL-positive strains. While not yet approved for clinical use, CEP-ZID’s potential as a weapon against MDR Gram-negative bacteria is significant. The bacterial susceptibility and resistance profile (CEP–ZID) [64], [65] is presented in Table 11 [Tab. 11] and Table 12 [Tab. 12].
Table 11: Cefepime/Zidebactam susceptibility in key bacterial populations (modified according to [65])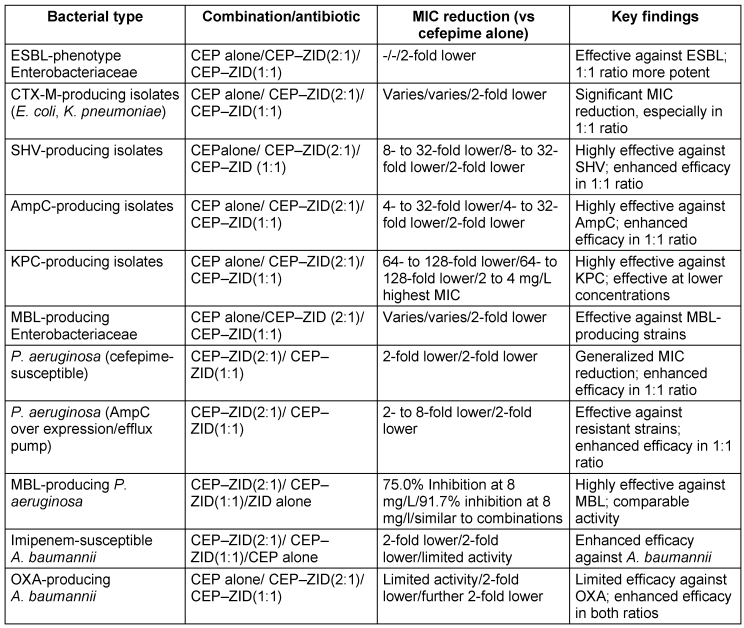
Table 12: Resistance profile of cefepime-zidebactam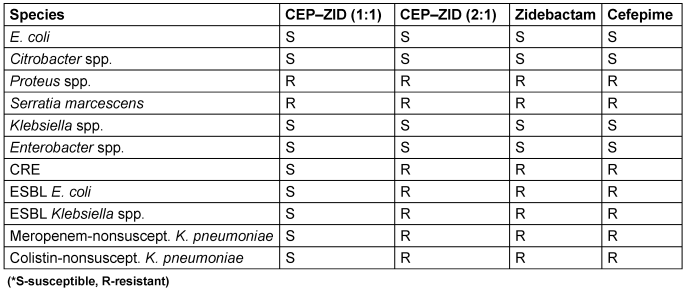
Pharmacokinetic and pharmacodynamic characteristics
The pharmacokinetic analysis provided by the manufacturer for WCK 5222, indicates that ZID has a half-life of 1.84 to 2.39 hours, while CEP has an average half-life of two hours. Both drugs are primarily excreted through the kidneys. ZID shows dose-proportional increases in AUC0-∞ and Cmax, whereas CEP exhibits linear pharmacokinetics across different doses. These findings offer valuable insights into the clinical dosing and safety of WCK 5222. However, no human PK-PD studies have been published for the CEP-ZID combination.
Efficacy and safety
Carbapenem-resistant P. aeruginosa infections, particularly those involving MBL-producing strains, are challenging to treat due to limited therapeutic options and high mortality rates. Traditional treatments like polymyxins are often hampered by poor pharmacokinetics and significant side effects. In one case involving an extensively drug-resistant (XDR) P. aeruginosa producing NDM, conventional therapies worsened the condition. However, the successful use of WCK 5222 as salvage therapy in a patient with acute T-cell leukemia demonstrated its efficacy, with the treatment showing consistent activity against XDR strains, including MBL producers, and resulting in a positive outcome with no adverse events [66]. Additionally, another case report highlights the compassionate use of WCK 5222 for treating a drug-resistant NDM-expressing P. aeruginosa infection in a patient with intra-abdominal infection-induced sepsis [67]. The use of CEP/ZID has also been reported for the successful treatment of sino-pulmonary infections and skull-based osteomyelitis caused by NDM-producing P. aeruginosa in a renal transplant recipient [68]; vide supra for its efficacy against resistant strains. Its efficacy in pediatric patients also holds promising hope in the treatment of PAN drug-resistant P. aeruginosa in lung empyema [69]. Furthermore, its in-vivo efficacy against carbapenem-resistant A. baumannii has been demonstrated in neutropenic murine pneumonia and thigh infection models [70], [71], [72], [73]. Studies have also reported its in-vivo activity against K. pneumoniae producing KPC and OXA-48-like enzymes in murine models [74].
4. Cefepime–enmetazobactam (CEP-ENZ)
In the ongoing global fight against antibiotic-resistant bacteria, particularly enterobacteriaceae, ENZ – formerly known as AAI101 – stands as a ground-breaking weapon. Driven by ESBLs, third-generation cephalosporin (3GC)-resistant Enterobacteriaceae are a major priority pathogen recognized by the World Health Organization. Each year, they cause an astounding 50 million severe infections globally [75], [76]. Class A β-lactamases that are resistant to tazobactam are successfully defeated by the new β-lactamase inhibitor ENZ, which works through a unique mechanism. The dynamic pairing of ENZ and the fourth-generation cephalosporin CEPdemonstrates potency comparable to meropenem and outperforms piperacillin-tazobactam against recent clinical isolates of enterobacteriaceae [76], [77]. As an empirical treatment to spare carbapenem usage, CEP-ENZ is effective against organisms coproducing OXA-48 β-lactamases and AmpC, especially in areas where ESBL-producing bacteria are common [78], [79].
The FDA, EMA and CMHP have approved Exblifep (Orchid Pharma), a CEP-ENZ combination to treat cUTIs in people ≥18 years of age, which is a noteworthy development in the battle against antibiotic resistance. The superiority of ENZ over piperacillin-tazobactam both in terms of clinical cure and microbiological eradication represents a major milestone, resulting in its historical approval. This achievement was observed during a worldwide phase-III study, highlighting the efficacy and potential impact of ENZ in combating antibiotic-resistant infections. It has an excellent success rate of 79.1% and a safety profile like that of piperacillin-tazobactam (58.9%) [80], [81], [82].
Mode of action
ENZ is a newly developed β-lactamase inhibitor with improved bacterial cell penetration and potency, attributed to the presence of a single methyl group, which differentiates it from tazobactam. Its neutral charge enhances its ability to penetrate the bacterial cell wall more effectively. The CEP-ENZ combination selectively inhibits many class A β-lactamases, including CTX-M, TEM, KPC and SHV. On the other hand, it does not obstruct carbapenemases or class D β-lactamases. When used alone, ENZ has no inhibitory effect on Gram-negative bacteria. The combination has shown in-vitro effectiveness against AmpC- and ESBL-producing Enterobacterales, as well as P. aeruginosa, with CEP being the key contributor to this activity [83]. ENZ dramatically increases cefepime’s therapeutic effectiveness, according to in vivo trials conducted on a mouse model of septicemia [84]. This combination offers a possible therapeutic option and supports “carbapenem sparing” approaches for infections caused by enterobacterales that produce ESBLs. Notably, ENZ has no antibacterial action against S. Maltophilia or A. baumannii [85].
Bacterial susceptibility and resistance profile
ENZ significantly enhances CEP’s potency against Enterobacteriaceae, particularly K. pneumoniae and E. coli, reducing MIC values by up to eightfold. While its efficacy against P. aeruginosa is limited, ENZ demonstrates superiority over tazobactam, especially for ESBL-producing K. pneumoniae. Clinical data reveal high susceptibility rates for CEP/ENZ against enterobacteriaceae, reaching 98.1%, with promising activity against ESBL-producing strains [86], [87], [88], [89]. However, its effectiveness against KPC-producing organisms remains limited. CEP/ENZ maintains high susceptibility rates (98.3% to 98.8%) even against 3GC-resistant strains, exhibiting robust activity against diverse resistance mechanisms such as ESBLs (susceptibility rates: 98.9% to 99.9%) [90]. An overview of regional disparities in Enterobacterales susceptibility to CEP/ENZ is presented in Table 13 [Tab. 13]. Clinical trials for cUTIs reveal a complex efficacy profile, with susceptibility varying due to the presence of multiple beta-lactamase genes and resistance mechanisms. No cross-resistance with non-beta-lactam antibiotics has been noted, suggesting a potential option for carbapenem- and cephalosporin-resistant infections. However, susceptibility testing remains essential for treatment decisions [91].
Table 13: Geographic variations in the susceptibility of Enterobacterales to cefepime enmetazobactam (modified according to [90])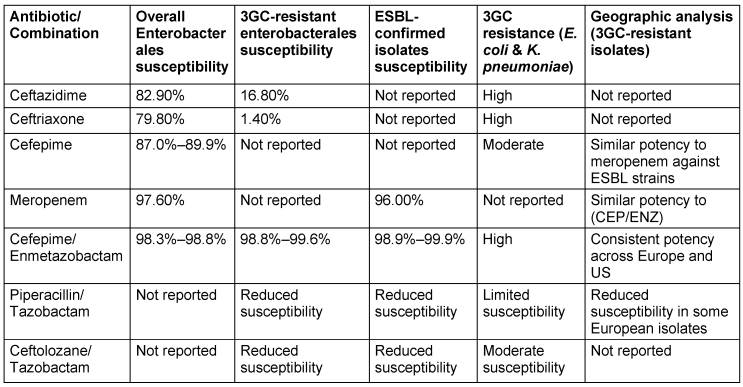
Pharmacokinetic and pharmacodynamic characteristics
The PK profiles of CEP and ENZ followed a standard two-compartment model with time-delineated intravenous input and first-order clearance. However, a prolonged terminal gamma phase of drug elimination was noted for CEP, requiring the addition of a third compartment for accurate representation of drug concentrations at later time points. In murine models, both drugs readily penetrate the epithelial lining fluid (ELF) of the lungs, without evidence of system hysteresis. Combination therapy with CEP/ENZ showed promising efficacy, especially against K. pneumoniae, with dose-response relationships established through murine experiments. Fractionation studies revealed no significant differences in antibacterial activity across different ENZ schedules. These findings support the potential of CEP/ENZ as a therapeutic option against multidrug-resistant pathogens, highlighting the importance of considering pharmacokinetics and pharmacodynamics in treatment optimization [92]. The PKPD characteristics of CEP/ENZ are discussed in Table 14 [Tab. 14], and the key aspects of PK-PD are articulated in Table 15 [Tab. 15].
Table 14: Pharmacokinetic parameters based on murine model data (modified according to [92])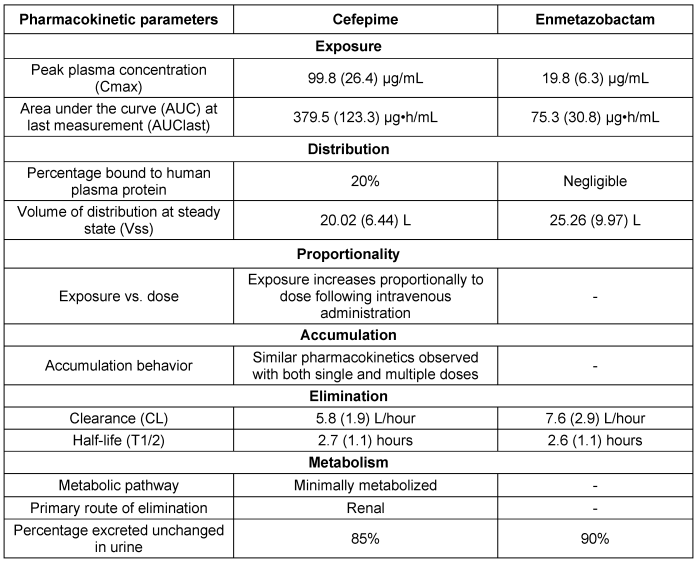
Table 15: Key aspects of PK-PD of cefepime-enmetazobactam (modified according to [92])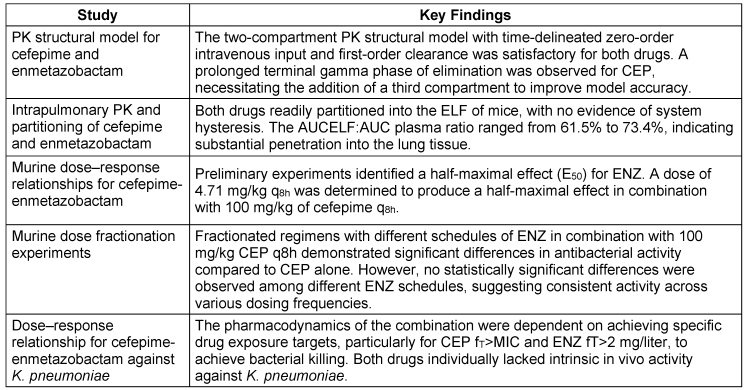
Safety profile and adverse events
The adverse effects of CEP-ENZ share many of the common side-effects seen with other β-lactam/β-lactamase inhibitor (BL/BLI) combinations such as Avycaz®, Emblaveo®, or Zerbaxa®. The combination has significantly advanced the treatment of cUTIs and pneumonia in adults, although its use requires careful safety assessment by healthcare providers. While generally well tolerated, the combination may lead to serious adverse reactions, particularly in patients with a history of severe hypersensitivity to CEP or other beta-lactam antibiotics. Neurological complications, such as encephalopathy or seizures, are possible, especially in individuals with renal dysfunction, necessitating dose adjustments. Vigilance for C. difficile-associated diarrhoea (CDAD) is crucial because of the risk associated with antibiotic use. Other safety considerations include the potential for positive Coombs’ tests, alterations in blood clotting time, and the emergence of drug-resistant bacteria. Although clinical trials have reported manageable side effects such as elevated liver enzymes and mild allergic reactions, caution is advised in elderly patients and those with renal impairment, who may require potential dose adjustments. In the case of overdose, supportive care is recommended, with hemodialysis as a possible option to remove excess drug. Healthcare professionals should thoroughly evaluate potential adverse effects and patient suitability before prescribing the CEP/ENZ combination [91].
5. Cefepime–taniborbactam
Taniborbactam, initially known as VNRX-5133, was developed by Venatorx Pharmaceuticals in 2014, with a patent filed that same year. It is a cyclic boronate BLI, designed to combat antibiotic-resistant bacteria. Cefepime-taniborbactam, an investigational intravenous antibiotic combination, pairs CEP, a fourth-generation cephalosporin, with taniborbactam, a bicyclic boronate BLI that has a broad inhibitory profile against both serine-based and MBLs. This combination is being developed to treat HABP, VABP and cUTIs, including pyelonephritis. The U.S. FDA has accepted its new drug application and set a PDUFA date of February 22, 2024, particularly for review in the treatment of cUTIs.
A key feature of taniborbactam is its activity against various MBLs, unlike other BLIs. This strength, however, comes with a caveat – it should be reserved for cases where piperacillin/tazobactam or CEP/ENZ is not appropriate, and only if ceftazidime-avibactam proves ineffective. Multidrug-resistant Gram-negative bacteria, especially carbapenem-resistant Enterobacterales and P. aeruginosa, present a significant healthcare challenge due to resistance mechanisms, such as beta-lactamase production. Research has demonstrated taniborbactam’s broad inhibitory efficacy, restoring CEP’s antibacterial activity and offering a promising therapeutic option for combating MDR Gram-negative bacterial infections [93], [94], [95], [96].
Mode of action
CEP acts by inhibiting PBPs, particularly PBP3. PBPs are crucial enzymes responsible for cross-linking peptidoglycan polymers, which are vital components of the bacterial cell wall. By specifically binding to PBP3, CEP disrupts this cross-linking process, hindering the formation of a structurally sound cell wall. This ultimately leads to cell-wall instability and bacterial cell death.
Tanibactam, a potent beta-lactamase inhibitor, addresses a key resistance mechanism employed by MDR bacteria. Beta-lactamases are enzymes produced by some bacteria that can hydrolyse (breakdown) beta-lactam antibiotics, rendering them ineffective. Tanibactam specifically inhibits these beta-lactamases, preventing the degradation of CEP. This ensures that the antibiotic retains its bactericidal activity against MDR bacteria that otherwise possess beta-lactamase-mediated resistance [96].
The synergy between CEP and taniborbactam lies in their coordinated action against MDR bacteria. While CEP directly targets the bacterial cell wall, MDR bacteria may employ beta-lactamases to counter its effects. By inhibiting these beta-lactamases, tanibactam interferes with the bacterial defense mechanism. This enables CEP to exert its full bactericidal effect, overcoming resistance mechanisms and efficiently eliminating MDR bacteria. Together, CEP and taniborbactam form a potent combination, with CEP disrupting cell-wall synthesis and taniborbactam, ensuring its efficacy by preventing breakdown. This coordinated approach offers a significant advantage in combating MDR Gram-negative infections, effectively addressing established resistance mechanisms [96].
Bacterial susceptibility and resistance profile
Antimicrobial susceptibility studies have revealed alarming patterns of resistance in different bacterial phenotypes. CEP/taniborbactam is highly effective, showing near-universal susceptibility across studied multidrug-resistant bacterial strains. This is a positive advancement in the battle against antibiotic resistance (AMR). On the other hand, there has been an increase in resistance to well-known antibiotics, such as ceftolozane-tazobactam and piperacillin-tazobactam, especially in populations with ESBL or MDR phenotypes. This emphasizes how AMR is becoming a larger problem and how urgently new antibiotic drugs need to be researched and developed. The in-vitro susceptibility of multidrug-resistant Enterobacterales to CEP/taniborbactam compared with established antibiotics is depicted in Figure 2 [Fig. 2].
Figure 2: Average bacterial susceptibility toantimicrobials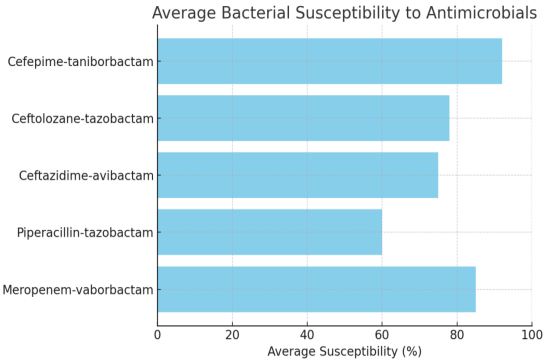
A recent study by Karlowsky et al. [97] evaluated the efficacy of CEP/taniborbactam against MDR Gram-negative bacteria. Conducted by IHMA and Venatorx Pharmaceuticals, the study analyzed strains of carbapenem-resistant Enterobacterales (CRE) and carbapenem-resistant Pseudomonas aeruginosa (CRPA) isolated from patients between 2018 and 2020. The results demonstrated a significant improvement in susceptibility rates compared to conventional β-lactam/β-lactamase inhibitor combinations, including ceftazidime-avibactam, ceftolozane-tazobactam, meropenem-vaborbactam, and piperacillin-tazobactam. Notably, CEP/taniborbactam exhibited greater than a 64-fold increased potency against Enterobacterales, with 99.7% of isolates inhibited at 16 mg/mL. Similar effectiveness was observed against P. aeruginosa, with a 4-fold increase in susceptibility and over 97% inhibition at the same concentration, even against strains with the VIM carbapenemase enzyme. The key bacterial susceptibility and resistance profiles are discussed in Table 16 [Tab. 16].
Table 16: Cefepime-taniborbactam susceptibility in key bacterial populations and resistance profiles (modified according to [97])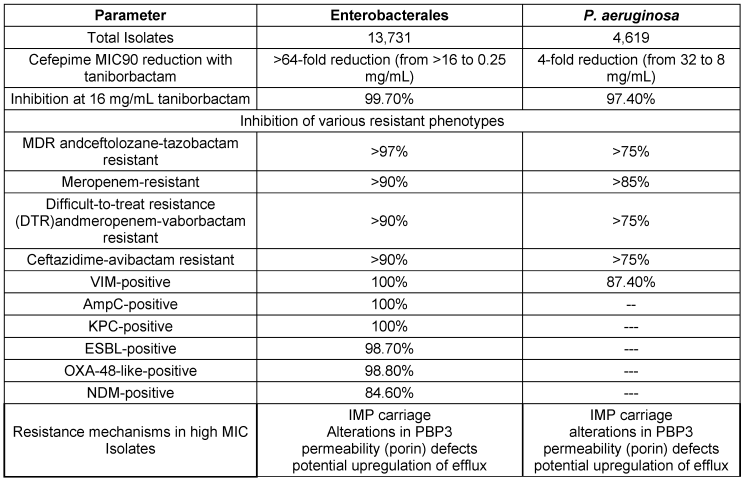
Pharmacokinetic and pharmacodynamic characteristics
Lasko et al. [98] demonstrated significant bacterial eradication in a neutropenic murine model of cUTI with CEP/taniborbactam, even against CEP-resistant clinical isolates harboring various enzymes, with an MIC of 32 mg/L. Human trials, including a phase-III study for cUTI treatment and safety assessment in healthy individuals, are ongoing [99]. Dowell et al. [100] found that taniborbactam when administered in human subjects exhibited dose-proportional pharmacokinetics, minimal accumulation, ~90% renal elimination, and good tolerability with no serious adverse events. A study focusing on lung penetration and efficacy against pneumonia analyzed the PK-PD profile of CEP/taniborbactam in twenty participants. Comparable plasma concentrations of taniborbactam and CEP before and after the third dose resulted in steady-state concentrations [101]. The findings of pharmacokinetics in plasma, ELF, and alveolar macrophages (AM) from a bronchoscopy study involving 20 participants are presented in Table 17 [Tab. 17].
Table 17: Cefepime-taniborbactam pharmacokinetics in plasma, epithelial lining fluid, and alveolar macrophages from a bronchoscopy study (n=20) (modified according to [101])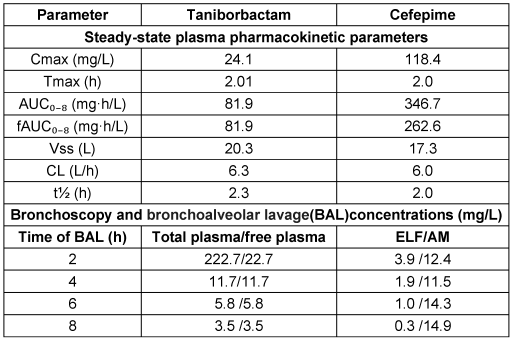
Taniborbactam exhibited a 100% unbound fraction in plasma, with steady-state fAUC0–8 values consistent with previous phase-I findings. Cefepime, in contrast, showed a variable unbound fraction. Regarding lung penetration, taniborbactam demonstrated modest distribution into ELF and AM, with approximately 17% penetration efficiency into ELF and peak AM concentrations at 8 hours. For pharmacodynamics, the fAUC0–24:MIC ratio in free plasma is critical for taniborbactam’s efficacy against MDR pathogens. Abdelraouf et al. [102] identified the optimal PK/PD indices for cefepime-taniborbactam in murine thigh infection models, demonstrating that dosing frequency did not impact taniborbactam’s potentiation of cefepime, with fAUC0–24:MIC values supporting significant bacterial reduction against Enterobacterales and P. aeruginosa, and the HSR dose (0.5 g q8h) achieving ≥1 log kill against all test isolates.
Safety profile and adverse events associated
A recent clinical safety evaluation investigated the tolerability of CEP/taniborbactam, a promising antibiotic combination that targets MDR Gram-negative bacteria. The study included 20 subjects who received three doses of the drug. Notably, co-administration was well tolerated, with no reports of serious adverse events or fatalities. Treatment-emergent adverse events (TEAEs) were observed in 14 subjects, with the most frequent being a temporary increase in white blood cell count (leucocytosis), which affected six participants. Other TEAEs reported by at least two subjects included mild elevations in bilirubin levels, dizziness, chills, slight changes in kidney function, and variations in blood clotting parameters. Importantly, all TEAEs were classified as mild in severity, and no subject discontinued the study because of adverse effects [100].
Additionally, the bronchoscopy and bronchoalveolar lavage procedures used for lung assessment were well tolerated by all participants, with only two requiring minimal conscious sedation. These findings contribute to the growing body of evidence supporting a favorable safety profile for CEP/taniborbactam, suggesting its potential as a safe and effective therapeutic option for patients battling serious infections caused by MDR Gram-negative bacteria [99].
Conclusion
The emergence of MDR Gram-negative bacteria, particularly with the rise of ESBLs, carbapenemases, and other β-lactamases, presents a significant challenge to antibiotic therapy. However, the development of novel β-lactam/β-lactamase inhibitor (BLBLI) combinations such as cefepime-zidebactam, cefepime-enmetazobactam, and cefepime-taniborbactam offers promising solutions. These combinations demonstrate potent antibacterial activity against various resistance mechanisms, providing effective treatment options for challenging pathogens such as Enterobacterales, P. aeruginosa, and A. baumannii. They also offer a carbapenem-sparing alternative for common infections caused by ESBL/AmpC-producing Enterobacterales and non-carbapenem-resistant P. aeruginosa. Despite their high cost, current recommendations suggest their use as definitive therapy for resistant isolates, with specific combinations preferred for certain resistant enterobacterales. Nevertheless, ongoing research and development of β-lactamase inhibitors are crucial to address the evolving landscape of antibiotic resistance. The emergence of new classes of β-lactamase inhibitors holds promise for protecting valuable antibiotics and overcoming resistance mechanisms. Overall, the development and implementation of novel BLBLI combinations represent significant progress in combating MDR Gram-negative bacteria, but continued efforts are essential to overcome emerging resistance threats.
Notes
Authors’ ORCIDs
Marzooka KC: 0000-0002-6103-7806
Dehghan MH: 0000-0002-8082-9454
Bilal K: 0009-0000-8071-6268
Imman R: 0000-0001-9969-1128
Funding
None.
Competing interests
The authors declare that they have no competing interests.
References
[1] Bush K, Bradford PA. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb Perspect Med. 2016 Aug 1;6(8):a025247. DOI: 10.1101/cshperspect.a025247[2] Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VHA, Takebayashi Y, Spencer J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J Mol Biol. 2019 Aug;431(18):3472-500. DOI: 10.1016/j.jmb.2019.04.002
[3] Khanna NR, Gerriets V. Beta Lactamase Inhibitors. In: StatPearls [Internet]. Treasure Island, FL, USA:StatPearls Publishing; 2021. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557592
[4] van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017 May;8(4):460-9. DOI: 10.1080/21505594.2016.1222343
[5] Doi Y, Paterson DL. Carbapenemase-producing Enterobacteriaceae. Semin Respir Crit Care Med. 2015 Feb;36(1):74-84. DOI: 10.1055/s-0035-1544208
[6] Goffin C, Ghuysen JM. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol Mol Biol Rev. 1998 Dec;62(4):1079-93. DOI: 10.1128/MMBR.62.4.1079-1093.1998
[7] Yocum RR, Waxman DJ, Rasmussen JR, Strominger JL. Mechanism of penicillin action: penicillin and substrate bind covalently to the same active site serine in two bacterial D-alanine carboxypeptidases. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2730-4. DOI: 10.1073/pnas.76.6.2730
[8] Lohans CT, Chan HTH, Malla TR, Kumar K, Kamps JJAG, McArdle DJB, van Groesen E, de Munnik M, Tooke CL, Spencer J, Paton RS, Brem J, Schofield CJ. Non-Hydrolytic β-Lactam Antibiotic Fragmentation by l,d-Transpeptidases and Serine β-Lactamase Cysteine Variants. Angew Chem Int Ed Engl. 2019 Feb;58(7):1990-4. DOI: 10.1002/anie.201809424
[9] Lu Z, Wang H, Zhang A, Liu X, Zhou W, Yang C, Guddat L, Yang H, Schofield CJ, Rao Z. Structures of Mycobacterium tuberculosis Penicillin-Binding Protein 3 in Complex with Five ß-Lactam Antibiotics Reveal Mechanism of Inactivation. Mol Pharmacol. 2020 Apr;97(4):287-94. DOI: 10.1124/mol.119.118042
[10] Chen X, Li Y, Bai K, Gu M, Xu X, Jiang N, Chen Y, Li J, Luo L. Class A Penicillin-Binding Protein C Is Responsible for Stress Response by Regulation of Peptidoglycan Assembly in Clavibacter michiganensis. Microbiol Spectr. 2022 Oct;10(5):e0181622. DOI: 10.1128/spectrum.01816-22
[11] Massova I, Mobashery S. Kinship and diversification of bacterial penicillin-binding proteins and beta-lactamases. Antimicrob Agents Chemother. 1998 Jan;42(1):1-17. DOI: 10.1128/AAC.42.1.1
[12] Curtis NA, Orr D, Ross GW, Boulton MG. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533-9. DOI: 10.1128/AAC.16.5.533
[13] Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev. 2008 Mar;32(2):361-85. DOI: 10.1111/j.1574-6976.2007.00095.x
[14] Spratt BG. Properties of the penicillin-binding proteins of Escherichia coli K12. Eur J Biochem. 1977 Jan;72(2):341-52. DOI: 10.1111/j.1432-1033.1977.tb11258.x
[15] Spratt BG. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247-60. DOI: 10.1099/00221287-129-5-1247
[16] Babic M, Hujer AM, Bonomo RA. What's new in antibiotic resistance? Focus on beta-lactamases. Drug Resist Updat. 2006 Jun;9(3):142-56. DOI: 10.1016/j.drup.2006.05.005
[17] Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012 Jun;27(2):128-42. DOI: 10.3904/kjim.2012.27.2.128
[18] Vasoo S, Barreto JN, Tosh PK. Emerging issues in gram-negative bacterial resistance: an update for the practicing clinician. Mayo Clin Proc. 2015 Mar;90(3):395-403. DOI: 10.1016/j.mayocp.2014.12.002
[19] Fisher JF, Mobashery S. β-Lactam Resistance Mechanisms: Gram-Positive Bacteria and Mycobacterium tuberculosis. Cold Spring Harb Perspect Med. 2016 May;6(5):a025221. DOI: 10.1101/cshperspect.a025221
[20] Bush K. Proliferation and significance of clinically relevant β-lactamases. Ann N Y Acad Sci. 2013 Jan;1277:84-90. DOI: 10.1111/nyas.12023
[21] Bonomo RA. β-Lactamases: A Focus on Current Challenges. Cold Spring Harb Perspect Med. 2017 Jan;7(1):a025239. DOI: 10.1101/cshperspect.a025239
[22] Ibrahim ME, Abbas M, Al-Shahrai AM, Elamin BK. Phenotypic Characterization and Antibiotic Resistance Patterns of Extended-Spectrum ß-Lactamase- and AmpC ß-Lactamase-Producing Gram-Negative Bacteria in a Referral Hospital, Saudi Arabia. Can J Infect Dis Med Microbiol. 2019 Jun 26;2019:6054694. DOI: 10.1155/2019/6054694
[23] Ambler RP. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May;289(1036):321-31. DOI: 10.1098/rstb.1980.0049
[24] Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010 Mar;54(3):969-76. DOI: 10.1128/AAC.01009-09
[25] Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995 Jun;39(6):1211-33. DOI: 10.1128/AAC.39.6.1211
[26] Doi Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin Infect Dis. 2019 Nov;69(Suppl 7):S565-S575. DOI: 10.1093/cid/ciz830
[27] Karam G, Chastre J, Wilcox MH, Vincent JL. Antibiotic strategies in the era of multidrug resistance. Crit Care. 2016 Jun;20(1):136. DOI: 10.1186/s13054-016-1320-7
[28] Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, Xie L, Yang C, Ma X, Li H, Li W, Zhang X, Liao K, Man S, Wang S, Wen H, Li B, Guo Z, Tian J, Pei F, Liu L, Zhang L, Zou C, Hu T, Cai J, Yang H, Huang J, Jia X, Huang W, Cao B, Wang H. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob Agents Chemother. 2018 Feb;62(2):e01882-17. DOI: 10.1128/AAC.01882-17
[29] Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment Options for Carbapenem-Resistant Enterobacteriaceae Infections. Open Forum Infect Dis. 2015 Apr;2(2):ofv050. DOI: 10.1093/ofid/ofv050
[30] Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019 Aug;25(8):943-50. DOI: 10.1016/j.cmi.2019.04.013
[31] Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front Microbiol. 2019 Jan 30;10:80. DOI: 10.3389/fmicb.2019.00080
[32] Kaye KS, Pogue JM. Infections Caused by Resistant Gram-Negative Bacteria: Epidemiology and Management. Pharmacotherapy. 2015 Oct;35(10):949-62. DOI: 10.1002/phar.1636
[33] US FDA. Avycaz (ceftazidime and avibactam) for injection, for intravenous use: US prescribing information. Reference ID: 3949759. 2018. Available from: https://www.accessdata.fda.gov
[34] Shirley M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs. 2018 Apr;78(6):675-92. DOI: 10.1007/s40265-018-0902-x
[35] Kuang H, Zhong C, Wang Y, Ye H, Ao K, Zong Z, Lv X. Clinical characteristics and outcomes of patients with multidrug-resistant Gram-negative bacterial infections treated with ceftazidime/avibactam. J Glob Antimicrob Resist. 2020 Dec;23:404-7. DOI: 10.1016/j.jgar.2020.10.023
[36] European Medicines Agency. Zavicefta: summary of product characteristics. Reference Number: EMA/369737/2020. 2018. Available from: http://www.ema.europa.eu
[37] Rains CP, Bryson HM, Peters DH. Ceftazidime. An update of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1995 Apr;49(4):577-617. DOI: 10.2165/00003495-199549040-00008
[38] Bonnefoy A, Dupuis-Hamelin C, Steier V, Delachaume C, Seys C, Stachyra T, Fairley M, Guitton M, Lampilas M. In vitro activity of AVE1330A, an innovative broad-spectrum non-beta-lactam beta-lactamase inhibitor. J Antimicrob Chemother. 2004 Aug;54(2):410-7. DOI: 10.1093/jac/dkh358
[39] Ehmann DE, Jahić H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A. 2012 Jul;109(29):11663-8. DOI: 10.1073/pnas.1205073109
[40] Stachyra T, Péchereau MC, Bruneau JM, Claudon M, Frère JM, Miossec C, Coleman K, Black MT. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob Agents Chemother. 2010 Dec;54(12):5132-8. DOI: 10.1128/AAC.00568-10
[41] Lahiri SD, Mangani S, Durand-Reville T, Benvenuti M, De Luca F, Sanyal G, Docquier JD. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob Agents Chemother. 2013 Jun;57(6):2496-505. DOI: 10.1128/AAC.02247-12
[42] Papp-Wallace KM, Bajaksouzian S, Abdelhamed AM, Foster AN, Winkler ML, Gatta JA, Nichols WW, Testa R, Bonomo RA, Jacobs MR. Activities of ceftazidime, ceftaroline, and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single β-lactamases. Diagn Microbiol Infect Dis. 2015 May;82(1):65-9. DOI: 10.1016/j.diagmicrobio.2015.02.003
[43] Aktaş Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2012 Jan;39(1):86-9. DOI: 10.1016/j.ijantimicag.2011.09.012
[44] Citron DM, Tyrrell KL, Merriam V, Goldstein EJ. In vitro activity of ceftazidime-NXL104 against 396 strains of beta-lactamase-producing anaerobes. Antimicrob Agents Chemother. 2011 Jul;55(7):3616-20. DOI: 10.1128/AAC.01682-10
[45] Rasmussen BA, Bush K, Tally FP. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997 Jan;24 Suppl 1:S110-20. DOI: 10.1093/clinids/24.supplement_1.s110
[46] Spiliopoulou I, Kazmierczak K, Stone GG. In vitro activity of ceftazidime/avibactam against isolates of carbapenem-non-susceptible Enterobacteriaceae collected during the INFORM global surveillance programme (2015-17). J Antimicrob Chemother. 2020 Feb;75(2):384-91. DOI: 10.1093/jac/dkz456
[47] Yasmin M, Fouts DE, Jacobs MR, Haydar H, Marshall SH, White R, D'Souza R, Lodise TP, Rhoads DD, Hujer AM, Rojas LJ, Hoyen C, Perez F, Edwards A, Bonomo RA. Monitoring Ceftazidime-Avibactam and Aztreonam Concentrations in the Treatment of a Bloodstream Infection Caused by a Multidrug-Resistant Enterobacter sp. Carrying Both Klebsiella pneumoniae Carbapenemase-4 and New Delhi Metallo-β-Lactamase-1. Clin Infect Dis. 2020 Aug;71(4):1095-8. DOI: 10.1093/cid/ciz1155
[48] Allergan, Inc. Avycaz (package insert). Irvine, CA:Allergan, Inc.; 2019.
[49] FDA. Ceftazidime-avibactam. Center for Drug Evaluation and Research. Approval package for application number 206494Orig1s000.AVYCAZ injection. Silver Spring, MD:FDA;2015.
[50] Sternbach N, Leibovici Weissman Y, Avni T, Yahav D. Efficacy and safety of ceftazidime/avibactam: a systematic review and meta-analysis. J Antimicrob Chemother. 2018 Aug;73(8):2021-9. DOI: 10.1093/jac/dky124
[51] Vickery SB, McClain D, Wargo KA. Successful Use of Ceftolozane-Tazobactam to Treat a Pulmonary Exacerbation of Cystic Fibrosis Caused by Multidrug-Resistant Pseudomonas aeruginosa. Pharmacotherapy. 2016 Oct;36(10):e154-e159. DOI: 10.1002/phar.1825
[52] Stokem K, Zuckerman JB, Nicolau DP, Wungwattana M, Sears EH. Use of ceftolozane-tazobactam in a cystic fibrosis patient with multidrug-resistant pseudomonas infection and renal insufficiency. Respir Med Case Rep. 2018;23:8-9. DOI: 10.1016/j.rmcr.2017.10.012
[53] Garazzino S, Altieri E, Silvestro E, Pruccoli G, Scolfaro C, Bignamini E. Ceftolozane/Tazobactam for Treating Children With Exacerbations of Cystic Fibrosis Due to Pseudomonas aeruginosa: A Review of Available Data. Front Pediatr. 2020 May 5;8:173. DOI: 10.3389/fped.2020.00173
[54] Farrell DJ, Flamm RK, Sader HS, Jones RN. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. Hospitals (2011-2012). Antimicrob Agents Chemother. 2013 Dec;57(12):6305-10. DOI: 10.1128/AAC.01802-13
[55] Takeda S, Ishii Y, Hatano K, Tateda K, Yamaguchi K. Stability of FR264205 against AmpC beta-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007 Nov;30(5):443-5. DOI: 10.1016/j.ijantimicag.2007.05.019
[56] FDA. Ceftolozane-tazobactam. Center for Drug Evaluation and Research. Approval package for application number 206829Orig1s000. Silver Spring, MD:FDA; 2014.
[57] Merck & Co., Inc. Zerbaxa (package insert). Kenilworth, NJ:Merck & Co., Inc; 2019.
[58] Arakawa S, Kawahara K, Kawahara M, Yasuda M, Fujimoto G, Sato A, Yokokawa R, Yoshinari T, Rhee EG, Aoyama N. The efficacy and safety of tazobactam/ceftolozane in Japanese patients with uncomplicated pyelonephritis and complicated urinary tract infection. J Infect Chemother. 2019 Feb;25(2):104-10. DOI: 10.1016/j.jiac.2018.10.009
[59] Mikamo H, Monden K, Miyasaka Y, Horiuchi T, Fujimoto G, Fukuhara T, Yoshinari T, Rhee EG, Shizuya T. The efficacy and safety of tazobactam/ceftolozane in combination with metronidazole in Japanese patients with complicated intra-abdominal infections. J Infect Chemother. 2019 Feb;25(2):111-6. DOI: 10.1016/j.jiac.2018.10.012
[60] Pogue JM, Kaye KS, Veve MP, Patel TS, Gerlach AT, Davis SL, Puzniak LA, File TM, Olson S, Dhar S, Bonomo RA, Perez F. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2020 Jul;71(2):304-10. DOI: 10.1093/cid/ciz816
[61] Bassetti M, Castaldo N, Cattelan A, Mussini C, Righi E, Tascini C, Menichetti F, Mastroianni CM, Tumbarello M, Grossi P, Artioli S, Carannante N, Cipriani L, Coletto D, Russo A, Digaetano M, Losito AR, Peghin M, Capone A, Nicolè S, Vena A; CEFTABUSE Study Group. Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents. 2019 Apr;53(4):408-15. DOI: 10.1016/j.ijantimicag.2018.11.001
[62] Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, Leibovici L. New β-Lactam-β-Lactamase Inhibitor Combinations. Clin Microbiol Rev. 2020 Dec;34(1):e00115-20. DOI: 10.1128/CMR.00115-20
[63] Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. Potent β-Lactam Enhancer Activity of Zidebactam and WCK 5153 against Acinetobacter baumannii, Including Carbapenemase-Producing Clinical Isolates. Antimicrob Agents Chemother. 2017 Nov;61(11):e01238-17. DOI: 10.1128/AAC.01238-17
[64] Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother. 2017 Jun;72(6):1696-1703. DOI: 10.1093/jac/dkx050
[65] Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. WCK 5222 (Cefepime-Zidebactam) Antimicrobial Activity against Clinical Isolates of Gram-Negative Bacteria Collected Worldwide in 2015. Antimicrob Agents Chemother. 2017 May;61(5):e00072-17. DOI: 10.1128/AAC.00072-17
[66] Egge SL, Lewis JS 2nd, Hakki M. Case Commentary: Successful Use of Cefepime/Zidebactam (WCK 5222) as a Salvage Therapy for the Treatment of Disseminated Extensively Drug-Resistant New Delhi Metallo-ß-Lactamase-Producing Pseudomonas aeruginosa Infection in an Adult Patient with Acute T-Cell Leukemia. Antimicrob Agents Chemother. 2023 Aug 17;67(8):e0066323. DOI: 10.1128/aac.00663-23
[67] Dubey D, Roy M, Shah TH, Bano N, Kulshrestha V, Mitra S, Sangwan P, Dubey M, Imran A, Jain B, Velmurugan A, Bakthavatchalam YD, Veeraraghavan B. Compassionate use of a novel β-lactam enhancer-based investigational antibiotic cefepime/zidebactam (WCK 5222) for the treatment of extensively-drug-resistant NDM-expressing Pseudomonas aeruginosa infection in an intra-abdominal infection-induced sepsis patient: a case report. Ann Clin Microbiol Antimicrob. 2023 Jul;22(1):55. DOI: 10.1186/s12941-023-00606-x
[68] Soman R, Sirsat R, Sunavala A, Punatar N, Mehta J, Rodrigues C, Veeraraghavan B. Successful treatment of sino-pulmonary infection & skull base osteomyelitis caused by New Delhi metallo-β-lactamase-producing Pseudomonas aeruginosa in a renal transplant recipient by using an investigational antibiotic cefepime/zidebactam (WCK 5222). Eur J Clin Microbiol Infect Dis. 2024 Feb 28. DOI: 10.1007/s10096-024-04791-1
[69] Santosh G, Narreddy S, Barigala R, Polati VR, Ramesh V. Cefepime/Zidebactum as a promising therapeutic option in the treatment of pan-drug resistant pseudomonas empyema, CIDSCON 2023 Selected Abstracts. J Clin Infect Dis Soc. 2023; 1(1):83. DOI: 10.4103/3333-5555.380637.
[70] Avery LM, Abdelraouf K, Nicolau DP. Assessment of the In Vivo Efficacy of WCK 5222 (Cefepime-Zidebactam) against Carbapenem-Resistant Acinetobacter baumannii in the Neutropenic Murine Lung Infection Model. Antimicrob Agents Chemother. 2018 Oct 24;62(11):e00948-18. DOI: 10.1128/AAC.00948-18
[71] Almarzoky Abuhussain SS, Avery LM, Abdelraouf K, Nicolau DP. In Vivo Efficacy of Humanized WCK 5222 (Cefepime-Zidebactam) Exposures against Carbapenem-Resistant Acinetobacter baumannii in the Neutropenic Thigh Model. Antimicrob Agents Chemother. 2018 Dec 21;63(1):e01931-18. DOI: 10.1128/AAC.01931-18
[72] Kidd JM, Abdelraouf K, Nicolau DP. Efficacy of human-simulated bronchopulmonary exposures of cefepime, zidebactam and the combination (WCK 5222) against MDR Pseudomonas aeruginosa in a neutropenic murine pneumonia model. J Antimicrob Chemother. 2020 Jan;75(1):149-55. DOI: 10.1093/jac/dkz414
[73] Monogue ML, Tabor-Rennie J, Abdelraouf K, Nicolau DP. In Vivo Efficacy of WCK 5222 (Cefepime-Zidebactam) against Multidrug-Resistant Pseudomonas aeruginosa in the Neutropenic Murine Thigh Infection Model. Antimicrob Agents Chemother. 2019 Jun 24;63(7):e00233-19. DOI: 10.1128/AAC.00233-19
[74] Lasko MJ, Abdelraouf K, Nicolau DP. Comparative in vivo activity of human-simulated plasma and epithelial lining fluid exposures of WCK 5222 (cefepime/zidebactam) against KPC- and OXA-48-like-producing Klebsiella pneumoniae in the neutropenic murine pneumonia model. J Antimicrob Chemother. 2021 Aug;76(9):2310-6. DOI: 10.1093/jac/dkab183
[75] Carcione D, Siracusa C, Sulejmani A, Leoni V, Intra J. Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use. Antibiotics (Basel). 2021 Aug;10(8):995. DOI: 10.3390/antibiotics10080995
[76] Gad El-Rab SMF, Halawani EM, Hassan A. Formulation of Ceftriaxone Conjugated Gold Nanoparticles and Their Medical Applications against Extended-Spectrum β-Lactamase Producing Bacteria and Breast Cancer. J Microbiol Biotechnol. 2018 Sep;28(9):1563-1572. DOI: 10.4014/jmb.1711.11037
[77] Kaye KS, Belley A, Barth P, Lahlou O, Knechtle P, Motta P, Velicitat P. Effect of Cefepime/Enmetazobactam vs Piperacillin/Tazobactam on Clinical Cure and Microbiological Eradication in Patients With Complicated Urinary Tract Infection or Acute Pyelonephritis: A Randomized Clinical Trial. JAMA. 2022 Oct;328(13):1304-1314. DOI: 10.1001/jama.2022.17034
[78] Papp-Wallace KM, Bethel CR, Caillon J, Barnes MD, Potel G, Bajaksouzian S, Rutter JD, Reghal A, Shapiro S, Taracila MA, Jacobs MR, Bonomo RA, Jacqueline C. Beyond Piperacillin-Tazobactam: Cefepime and AAI101 as a Potent β-Lactam-β-Lactamase Inhibitor Combination. Antimicrob Agents Chemother. 2019 May;63(5):. DOI: 10.1128/AAC.00105-19
[79] Morrissey I, Magnet S, Hawser S, Shapiro S, Knechtle P. In Vitro Activity of Cefepime-Enmetazobactam against Gram-Negative Isolates Collected from U.S. and European Hospitals during 2014-2015. Antimicrob Agents Chemother. 2019 Jun 24;63(7):e00514-19. DOI: 10.1128/AAC.00514-19
[80] Express Pharma. Orchid Pharma gets USFDA approval for Exblifep. 2024 [retrieved February 23, 2024]. Available from: https://www.expresspharma.in/orchid-pharma-gets-usfda-approval-for-exblifep/
[81] Business Wire. Allecra Therapeutics submits New Drug Application to the US FDA for EXBLIFEP for the treatment of complicated urinary tract infections. 2023 [retrieved February 23, 2024]. Available from: https://www.businesswire.com/news/home/20230627361506/en/Allecra-Therapeutics-Submits-New-Drug-Application-to-the-U.S.-FDA-for-EXBLIFEP%C2%AE-for-the-Treatment-of-Complicated-Urinary-Tract-Infections
[82] Allecra Therapeutics. Allecra Therapeutics announces positive top-line results for phase 3 ALLIUM clinical trial of EXBLIFEP for complicated Urinary Tract Infections. 2020 [retrieved February 23, 2024]. Available from: https://www.globenewswire.com/news-release/2020/02/25/1989893/0/en/Allecra-Therapeutics-Announces-Positive-Top-Line-Results-for-Phase-3-ALLIUM-Clinical-Trial-of-EXBLIFEP-for-Complicated-Urinary-Tract-Infections.html
[83] Isler B, Harris P, Stewart AG, Paterson DL. An update on cefepime and its future role in combination with novel β-lactamase inhibitors for MDR Enterobacterales and Pseudomonas aeruginosa. J Antimicrob Chemother. 2021 Feb;76(3):550-60. DOI: 10.1093/jac/dkaa511
[84] Zhang S, Liao X, Ding T, Ahn J. Role of β-Lactamase Inhibitors as Potentiators in Antimicrobial Chemotherapy Targeting Gram-Negative Bacteria. Antibiotics (Basel). 2024 Mar;13(3):260. DOI: 10.3390/antibiotics13030260
[85] Liu PY, Ko WC, Lee WS, Lu PL, Chen YH, Cheng SH, Lu MC, Lin CY, Wu TS, Yen MY, Wang LS, Liu CP, Shao PL, Lee YL, Shi ZY, Chen YS, Wang FD, Tseng SH, Lin CN, Chen YH, Sheng WH, Lee CM, Tang HJ, Hsueh PR. In vitro activity of cefiderocol, cefepime/enmetazobactam, cefepime/zidebactam, eravacycline, omadacycline, and other comparative agents against carbapenem-non-susceptible Pseudomonas aeruginosa and Acinetobacter baumannii isolates associated from bloodstream infection in Taiwan between 2018-2020. J Microbiol Immunol Infect. 2022 Oct;55(5):888-95. DOI: 10.1016/j.jmii.2021.08.012
[86] Bush K. Past and Present Perspectives on β-Lactamases. Antimicrob Agents Chemother. 2018 Oct;62(10):e01076-18. DOI: 10.1128/AAC.01076-18
[87] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 29th ed. CLSI supplement M100. Wayne, PA:CLSI; 2019.
[88] European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0. Basel, Switzerland:EUCAST; 2019.
[89] Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. Carbapenemase-Producing Organisms: A Global Scourge. Clin Infect Dis. 2018 Apr;66(8):1290-7. DOI: 10.1093/cid/cix893
[90] Belley A, Morrissey I, Hawser S, Kothari N, Knechtle P. Third-generation cephalosporin resistance in clinical isolates of Enterobacterales collected between 2016-2018 from USA and Europe: genotypic analysis of β-lactamases and comparative in vitro activity of cefepime/enmetazobactam. J Glob Antimicrob Resist. 2021 Jun;25:93-101. DOI: 10.1016/j.jgar.2021.02.031
[91] EXBLIFEP®. (cefepime and enmetazobactam) for injection, for intravenous use [Package insert]. Retrieved from Drugs @FDA [Database]. (Accession No. 5333107). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/216165s000lbl.pdf
[92] Johnson A, McEntee L, Farrington N, Kolamunnage-Dona R, Franzoni S, Vezzelli A, Massimiliano M, Knechtle P, Belley A, Dane A, Drusano G, Das S, Hope W. Pharmacodynamics of Cefepime Combined with the Novel Extended-Spectrum-β-Lactamase (ESBL) Inhibitor Enmetazobactam for Murine Pneumonia Caused by ESBL-Producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2020 May 21;64(6):e00180-20. DOI: 10.1128/AAC.00180-20
[93] Krajnc A, Brem J, Hinchliffe P, Calvopiña K, Panduwawala TD, Lang PA, Kamps JJAG, Tyrrell JM, Widlake E, Saward BG, Walsh TR, Spencer J, Schofield CJ. Bicyclic Boronate VNRX-5133 Inhibits Metallo- and Serine-β-Lactamases. J Med Chem. 2019 Sep;62(18):8544-56. DOI: 10.1021/acs.jmedchem.9b00911
[94] Liu B, Trout REL, Chu GH, McGarry D, Jackson RW, Hamrick JC, Daigle DM, Cusick SM, Pozzi C, De Luca F, Benvenuti M, Mangani S, Docquier JD, Weiss WJ, Pevear DC, Xerri L, Burns CJ. Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-β-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J Med Chem. 2020 Mar;63(6):2789-801. DOI: 10.1021/acs.jmedchem.9b01518
[95] Hamrick JC, Docquier JD, Uehara T, Myers CL, Six DA, Chatwin CL, John KJ, Vernacchio SF, Cusick SM, Trout REL, Pozzi C, De Luca F, Benvenuti M, Mangani S, Liu B, Jackson RW, Moeck G, Xerri L, Burns CJ, Pevear DC, Daigle DM. VNRX-5133 (Taniborbactam), a Broad-Spectrum Inhibitor of Serine- and Metallo-β-Lactamases, Restores Activity of Cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01963-19. DOI: 10.1128/AAC.01963-19
[96] VenatoRxPharmaceuticals. Cefepime-Taniborbactam. [retrieved March 21, 2024]. Available from: https://venatorx.com/press-releases/venatorx-announces-presentation-of-analysis-of-cefepime taniborbactam-in-vitro-activity-against-clinically-significant-gram-negative-bacteria-isolated-from patients-with-cancer-at-idweek-2023/
[97] Karlowsky JA, Hackel MA, Wise MG, Six DA, Uehara T, Daigle DM, Cusick SM, Pevear DC, Moeck G, Sahm DF. In Vitro Activity of Cefepime-Taniborbactam and Comparators against Clinical Isolates of Gram-Negative Bacilli from 2018 to 2020: Results from the Global Evaluation of Antimicrobial Resistance via Surveillance (GEARS) Program. Antimicrob Agents Chemother. 2023 Jan 24;67(1):e0128122. DOI: 10.1128/aac.01281-22
[98] Lasko MJ, Nicolau DP, Asempa TE. Clinical exposure-response relationship of cefepime/taniborbactam against Gram-negative organisms in the murine complicated urinary tract infection model. J Antimicrob Chemother. 2022 Feb;77(2):443-7. DOI: 10.1093/jac/dkab405
[99] Principe L, Lupia T, Andriani L, Campanile F, Carcione D, Corcione S, De Rosa FG, Luzzati R, Stroffolini G, Steyde M, Decorti G, Di Bella S. Microbiological, Clinical, and PK/PD Features of the New Anti-Gram-Negative Antibiotics: β-Lactam/β-Lactamase Inhibitors in Combination and Cefiderocol-An All-Inclusive Guide for Clinicians. Pharmaceuticals (Basel). 2022 Apr;15(4):463. DOI: 10.3390/ph15040463
[100] Dowell JA, Dickerson D, Henkel T. Safety and Pharmacokinetics in Human Volunteers of Taniborbactam (VNRX-5133), a Novel Intravenous β-Lactamase Inhibitor. Antimicrob Agents Chemother. 2021 Oct;65(11):e0105321. DOI: 10.1128/AAC.01053-21
[101] Asempa TE, Kuti JL, Nascimento JC, Pope SJ, Salerno EL, Troy PJ, Nicolau DP. Bronchopulmonary disposition of IV cefepime/taniborbactam (2-0.5 g) administered over 2 h in healthy adult subjects. J Antimicrob Chemother. 2023 Mar;78(3):703-9. DOI: 10.1093/jac/dkac447
[102] Abdelraouf K, Almarzoky Abuhussain S, Nicolau DP. In vivo pharmacodynamics of new-generation β-lactamase inhibitor taniborbactam (formerly VNRX-5133) in combination with cefepime against serine-β-lactamase-producing Gram-negative bacteria. J Antimicrob Chemother. 2020 Dec;75(12):3601-10. DOI: 10.1093/jac/dkaa373
[103] Sonda T, Kumburu H, van Zwetselaar M, Alifrangis M, Lund O, Kibiki G, Aarestrup FM. Meta-analysis of proportion estimates of Extended-Spectrum-Beta-Lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob Resist Infect Control. 2016;5:18. DOI: 10.1186/s13756-016-0117-4
[104] Onduru OG, Mkakosya RS, Aboud S, Rumisha SF. Genetic Determinants of Resistance among ESBL-Producing Enterobacteriaceae in Community and Hospital Settings in East, Central, and Southern Africa: A Systematic Review and Meta-Analysis of Prevalence. Can J Infect Dis Med Microbiol. 2021 Jun 2;2021:5153237. DOI: 10.1155/2021/5153237
[105] Varela MF, Stephen J, Lekshmi M, Ojha M, Wenzel N, Sanford LM, Hernandez AJ, Parvathi A, Kumar SH. Bacterial Resistance to Antimicrobial Agents. Antibiotics (Basel). 2021 May;10(5):593. DOI: 10.3390/antibiotics10050593
[106] Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Réville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. Kinetics of avibactam inhibition against Class A, C, and D β-lactamases. J Biol Chem. 2013 Sep;288(39):27960-71. DOI: 10.1074/jbc.M113.485979
[107] Lahiri SD, Walkup GK, Whiteaker JD, Palmer T, McCormack K, Tanudra MA, Nash TJ, Thresher J, Johnstone MR, Hajec L, Livchak S, McLaughlin RE, Alm RA. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother. 2015;70(6):1650-8. DOI: 10.1093/jac/dkv004
[108] Livermore DM, Mushtaq S, Barker K, Hope R, Warner M, Woodford N. Characterization of β-lactamase and porin mutants of Enterobacteriaceae selected with ceftaroline + avibactam (NXL104). J Antimicrob Chemother. 2012 Jun;67(6):1354-8. DOI: 10.1093/jac/dks079
[109] Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother. 2015 Sep;59(9):5324-30. DOI: 10.1128/AAC.00678-15
[110] Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. Mutations in blaKPC-3 That Confer Ceftazidime-Avibactam Resistance Encode Novel KPC-3 Variants That Function as Extended-Spectrum ß-Lactamases. Antimicrob Agents Chemother. 2017 Apr 24;61(5):e02534-16. DOI: 10.1128/AAC.02534-16
[111] Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob Agents Chemother. 2017 Feb 23;61(3):e02097-16. DOI: 10.1128/AAC.02097-16
[112] Karlowsky JA, Biedenbach DJ, Kazmierczak KM, Stone GG, Sahm DF. Activity of Ceftazidime-Avibactam against Extended-Spectrum- and AmpC β-Lactamase-Producing Enterobacteriaceae Collected in the INFORM Global Surveillance Study from 2012 to 2014. Antimicrob Agents Chemother. 2016 May;60(5):2849-57. DOI: 10.1128/AAC.02286-15
[113] Nichols WW, de Jonge BL, Kazmierczak KM, Karlowsky JA, Sahm DF. In Vitro Susceptibility of Global Surveillance Isolates of Pseudomonas aeruginosa to Ceftazidime-Avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother. 2016 Aug;60(8):4743-9. DOI: 10.1128/AAC.00220-16
[114] Sun L, Chen W, Li H, Li L, Zou X, Zhao J, Lu B, Li B, Wang C, Li H, Liu Y, Cao B. Phenotypic and genotypic analysis of KPC-51 and KPC-52, two novel KPC-2 variants conferring resistance to ceftazidime/avibactam in the KPC-producing Klebsiella pneumoniae ST11 clone background. J Antimicrob Chemother. 2020 Oct;75(10):3072-4. DOI: 10.1093/jac/dkaa241
[115] Grupper M, Sutherland C, Nicolau DP. Multicenter Evaluation of Ceftazidime-Avibactam and Ceftolozane-Tazobactam Inhibitory Activity against Meropenem-Nonsusceptible Pseudomonas aeruginosa from Blood, Respiratory Tract, and Wounds. Antimicrob Agents Chemother. 2017 Oct;61(10):e00875-17. DOI: 10.1128/AAC.00875-17
[116] Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother. 2010 Nov;65(11):2376-81. DOI: 10.1093/jac/dkq306
[117] Curcio D. Activity of a novel combination against multidrug-resistant nonfermenters: ceftazidime plus NXL104. Expert Rev Anti Infect Ther. 2011 Feb;9(2):173-6. DOI: 10.1586/eri.10.173
[118] Zhang Y, Kashikar A, Brown CA, Denys G, Bush K. Unusual Escherichia coli PBP 3 Insertion Sequence Identified from a Collection of Carbapenem-Resistant Enterobacteriaceae Tested In Vitro with a Combination of Ceftazidime-, Ceftaroline-, or Aztreonam-Avibactam. Antimicrob Agents Chemother. 2017 Jul 25;61(8):e00389-17. DOI: 10.1128/AAC.00389-17
[119] Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother. 2018 May;62(5):e02497-17. DOI: 10.1128/AAC.02497-17
[120] Bradley JS, Broadhurst H, Cheng K, Mendez M, Newell P, Prchlik M, Stone GG, Talley AK, Tawadrous M, Wajsbrot D, Yates K, Zuzova A, Gardner A. Safety and Efficacy of Ceftazidime-Avibactam Plus Metronidazole in the Treatment of Children ≥3 Months to <18 Years With Complicated Intra-Abdominal Infection: Results From a Phase 2, Randomized, Controlled Trial. Pediatr Infect Dis J. 2019 Aug;38(8):816-24. DOI: 10.1097/INF.0000000000002392
[121] Bradley JS, Roilides E, Broadhurst H, Cheng K, Huang LM, MasCasullo V, Newell P, Stone GG, Tawadrous M, Wajsbrot D, Yates K, Gardner A. Safety and Efficacy of Ceftazidime-Avibactam in the Treatment of Children ≥3 Months to <18 Years With Complicated Urinary Tract Infection: Results from a Phase 2 Randomized, Controlled Trial. Pediatr Infect Dis J. 2019 Sep;38(9):920-8. DOI: 10.1097/INF.0000000000002395
[122] Iosifidis E, Chorafa E, Agakidou E, Kontou A, Violaki A, Volakli E, Christou EI, Zarras C, Drossou-Agakidou V, Sdougka M, Roilides E. Use of Ceftazidime-avibactam for the Treatment of Extensively drug-resistant or Pan drug-resistant Klebsiella pneumoniae in Neonates and Children <5 Years of Age. Pediatr Infect Dis J. 2019 Aug;38(8):812-5. DOI: 10.1097/INF.0000000000002344
[123] Guimarães T, Nouér SA, Martins RCR, Perdigão Neto LV, Martins WMBS, Narciso Barbosa AC, Ferreira ALP, Costa SF, Gales AC. Ceftazidime-Avibactam as Salvage Therapy for Infections Caused by Enterobacteriales Coresistant to Carbapenems and Polymyxins. Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00528-19. DOI: 10.1128/AAC.00528-19
[124] King M, Heil E, Kuriakose S, Bias T, Huang V, El-Beyrouty C, McCoy D, Hiles J, Richards L, Gardner J, Harrington N, Biason K, Gallagher JC. Multicenter Study of Outcomes with Ceftazidime-Avibactam in Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother. 2017 Jul;61(7):e00449-17. DOI: 10.1128/AAC.00449-17
[125] Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Clin Infect Dis. 2016 Dec;63(12):1615-8. DOI: 10.1093/cid/ciw636
[126] De la Calle C, Rodríguez O, Morata L, Marco F, Cardozo C, García-Vidal C, Río AD, Feher C, Pellicé M, Puerta-Alcalde P, Mensa J, Soriano A, Martínez JA. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents. 2019 Apr;53(4):520-4. DOI: 10.1016/j.ijantimicag.2018.11.015
[127] Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program. Clin Infect Dis. 2016 Jun;62(11):1380-9. DOI: 10.1093/cid/ciw133
[128] Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, Chen Q, Song J, Laud PJ, Stone GG, Chow JW. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017 May;49(5):579-88. DOI: 10.1016/j.ijantimicag.2017.01.010
[129] Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, Yates K, Gasink LB. Ceftazidime-avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin Infect Dis. 2016 Sep;63(6):754-62. DOI: 10.1093/cid/ciw378
[130] Tamma PD, Fan Y, Bergman Y, Sick-Samuels AC, Hsu AJ, Timp W, Simner PJ, Prokesch BC, Greenberg DE. Successful Treatment of Persistent Burkholderia cepacia Complex Bacteremia with Ceftazidime-Avibactam. Antimicrob Agents Chemother. 2018 Apr;62(4):e02213-17. DOI: 10.1128/AAC.02213-17
[131] Daccò V, Claut L, Piconi S, Castellazzi L, Garbarino F, Teri A, Colombo C. Successful ceftazidime-avibactam treatment of post-surgery Burkholderia multivorans genomovar II bacteremia and brain abscesses in a young lung transplanted woman with cystic fibrosis. Transpl Infect Dis. 2019 Jun;21(3):e13082. DOI: 10.1111/tid.13082
[132] Los-Arcos I, Len O, Martín-Gómez MT, González-López JJ, Saéz-Giménez B, Deu M, Nuvials X, Ferrer R, Román A, Gavaldà J. Lung transplantation in two cystic fibrosis patients infected with previously pandrug-resistant Burkholderia cepacia complex treated with ceftazidime-avibactam. Infection. 2019 Apr;47(2):289-92. DOI: 10.1007/s15010-018-1261-y
[133] Spoletini G, Etherington C, Shaw N, Clifton IJ, Denton M, Whitaker P, Peckham DG. Use of ceftazidime/avibactam for the treatment of MDR Pseudomonas aeruginosa and Burkholderia cepacia complex infections in cystic fibrosis: a case series. J Antimicrob Chemother. 2019 May;74(5):1425-9. DOI: 10.1093/jac/dky558
[134] Cantón-Bulnes ML, Hurtado Martínez Á, López-Cerero L, Arenzana Seisdedos Á, Merino-Bohorquez V, Garnacho-Montero J. A case of pan-resistant Burkholderia cepacia complex bacteremic pneumonia, after lung transplantation treated with a targeted combination therapy. Transpl Infect Dis. 2019 Apr;21(2):e13034. DOI: 10.1111/tid.13034
[135] Barlow G, Morice A. Successful treatment of resistant Burkholderia multivorans infection in a patient with cystic fibrosis using ceftazidime/avibactam plus aztreonam. J Antimicrob Chemother. 2018 Aug;73(8):2270-1. DOI: 10.1093/jac/dky136
[136] Caverly LJ, Spilker T, Kalikin LM, Stillwell T, Young C, Huang DB, LiPuma JJ. In Vitro Activities of β-Lactam-β-Lactamase Inhibitor Antimicrobial Agents against Cystic Fibrosis Respiratory Pathogens. Antimicrob Agents Chemother. 2019 Dec;64(1):e01595-19. DOI: 10.1128/AAC.01595-19
[137] Deshpande D, Srivastava S, Chapagain ML, Lee PS, Cirrincione KN, Pasipanodya JG, Gumbo T. The discovery of ceftazidime/avibactam as an anti-Mycobacterium avium agent. J Antimicrob Chemother. 2017 Sep;72(suppl_2):i36-i42. DOI: 10.1093/jac/dkx306
[138] Deshpande D, Srivastava S, Pasipanodya JG, Lee PS, Gumbo T. A novel ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM) regimen for pulmonary Mycobacterium avium disease. J Antimicrob Chemother. 2017 Sep;72(suppl_2):i48-i53. DOI: 10.1093/jac/dkx307
[139] Deshpande D, Srivastava S, Chapagain M, Magombedze G, Martin KR, Cirrincione KN, Lee PS, Koeuth T, Dheda K, Gumbo T. Ceftazidime-avibactam has potent sterilizing activity against highly drug-resistant tuberculosis. Sci Adv. 2017 Aug;3(8):e1701102. DOI: 10.1126/sciadv.1701102
[140] Sutherland CA, Nicolau DP. Susceptibility Profile of Ceftolozane/Tazobactam and Other Parenteral Antimicrobials Against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa From US Hospitals. Clin Ther. 2015 Jul;37(7):1564-71. DOI: 10.1016/j.clinthera.2015.05.501
[141] Carvalhaes CG, Castanheira M, Sader HS, Flamm RK, Shortridge D. Antimicrobial activity of ceftolozane-tazobactam tested against gram-negative contemporary (2015-2017) isolates from hospitalized patients with pneumonia in US medical centers. Diagn Microbiol Infect Dis. 2019 May;94(1):93-102. DOI: 10.1016/j.diagmicrobio.2018.11.021
[142] Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12). J Antimicrob Chemother. 2014 Oct;69(10):2713-22. DOI: 10.1093/jac/dku184
[143] Pfaller MA, Barry AL, Fuchs PC, Gerlach EH, Hardy DJ, McLaughlin JC. Comparison of fixed concentration and fixed ratio options for dilution susceptibility testing of gram-negative bacilli to ampicillin and ampicillin/sulbactam. Eur J Clin Microbiol Infect Dis. 1993 May;12(5):356-62. DOI: 10.1007/BF01964434
[144] Shortridge D, Duncan LR, Pfaller MA, Flamm RK. Activity of ceftolozane-tazobactam and comparators when tested against Gram-negative isolates collected from paediatric patients in the USA and Europe between 2012 and 2016 as part of a global surveillance programme. Int J Antimicrob Agents. 2019 May;53(5):637-43. DOI: 10.1016/j.ijantimicag.2019.01.015
[145] Yin D, Wu S, Yang Y, Shi Q, Dong D, Zhu D, Hu F; China Antimicrobial Surveillance Network (CHINET) Study Group. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the In Vitro Activities of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Clinical Isolates of Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019 Mar 27;63(4):e02431-18. DOI: 10.1128/AAC.02431-18
[146] Tato M, García-Castillo M, Bofarull AM, Cantón R; CENIT Study Group. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacteriaceae recovered in Spanish medical centres: Results of the CENIT study. Int J Antimicrob Agents. 2015 Nov;46(5):502-10. DOI: 10.1016/j.ijantimicag.2015.07.004
[147] Pazzini C, Ahmad-Nejad P, Ghebremedhin B. Ceftolozane/Tazobactam Susceptibility Testing in Extended-Spectrum Betalactamase- and Carbapenemase-Producing Gram-Negative Bacteria of Various Clonal Lineages. Eur J Microbiol Immunol (Bp). 2019 Mar;9(1):1-4. DOI: 10.1556/1886.2019.00001
[148] Haidar G, Philips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, Doi Y, Hao B, Press EG, Cooper VS, Clancy CJ, Nguyen MH. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin Infect Dis. 2017 Jul;65(1):110-20. DOI: 10.1093/cid/cix182
[149] Skoglund E, Abodakpi H, Rios R, Diaz L, De La Cadena E, Dinh AQ, Ardila J, Miller WR, Munita JM, Arias CA, Tam VH, Tran TT. In Vivo Resistance to Ceftolozane/Tazobactam in Pseudomonas aeruginosa Arising by AmpC- and Non-AmpC-Mediated Pathways. Case Rep Infect Dis. 2018 Dec 23;2018:9095203. DOI: 10.1155/2018/9095203
[150] Fraile-Ribot PA, Cabot G, Mulet X, Periañez L, Martín-Pena ML, Juan C, Pérez JL, Oliver A. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J Antimicrob Chemother. 2018 Mar;73(3):658-63. DOI: 10.1093/jac/dkx424
[151] Barnes MD, Taracila MA, Rutter JD, Bethel CR, Galdadas I, Hujer AM, Caselli E, Prati F, Dekker JP, Papp-Wallace KM, Haider S, Bonomo RA. Deciphering the Evolution of Cephalosporin Resistance to Ceftolozane-Tazobactam in Pseudomonas aeruginosa. mBio. 2018 Dec;9(6):e02085-18. DOI: 10.1128/mBio.02085-18
[152] So W, Shurko J, Galega R, Quilitz R, Greene JN, Lee GC. Mechanisms of high-level ceftolozane/tazobactam resistance in Pseudomonas aeruginosa from a severely neutropenic patient and treatment success from synergy with tobramycin. J Antimicrob Chemother. 2019 Jan;74(1):269-71. DOI: 10.1093/jac/dky393
[153] Cabot G, Bruchmann S, Mulet X, Zamorano L, Moyà B, Juan C, Haussler S, Oliver A. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother. 2014 Jun;58(6):3091-9. DOI: 10.1128/AAC.02462-13
[154] Jorgensen SCJ, Trinh TD, Zasowski EJ, Lagnf AM, Simon SP, Bhatia S, Melvin SM, Steed ME, Finch NA, Morrisette T, Estrada SJ, Rosenberg JR, Davis SL, Rybak MJ. Real-World Experience with Ceftolozane-Tazobactam for Multidrug-Resistant Gram-Negative Bacterial Infections. Antimicrob Agents Chemother. 2020 Mar;64(4):e02291-19. DOI: 10.1128/AAC.02291-19
[155] Buonomo AR, Maraolo AE, Scotto R, Foggia M, Zappulo E, Congera P, Parente S, Gentile I. Efficacy and safety of ceftolozane/tazobactam as therapeutic option for complicated skin and soft tissue infections by MDR/XDR Pseudomonas aeruginosa in patients with impaired renal function: a case series from a single-center experience. Infection. 2020 Apr;48(2):303-7. DOI: 10.1007/s15010-020-01390-y
[156] Larson KB, Patel YT, Willavize S, Bradley JS, Rhee EG, Caro L, Rizk ML. Ceftolozane-Tazobactam Population Pharmacokinetics and Dose Selection for Further Clinical Evaluation in Pediatric Patients with Complicated Urinary Tract or Complicated Intra-abdominal Infections. Antimicrob Agents Chemother. 2019 Jun;63(6):e02578-18. DOI: 10.1128/AAC.02578-18
[157] Bradley JS, Ang JY, Arrieta AC, Larson KB, Rizk ML, Caro L, Yang S, Yu B, Johnson MG, Rhee EG. Pharmacokinetics and Safety of Single Intravenous Doses of Ceftolozane/Tazobactam in Children With Proven or Suspected Gram-Negative Infection. Pediatr Infect Dis J. 2018 Nov;37(11):1130-6. DOI: 10.1097/INF.0000000000002170
[158] ClinicalTrials.gov. MK-7625A versus meropenem in pediatric participants with complicated urinary tract infection (cUTI) (MK-7625A034). 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03230838
[159] Zikri A, El Masri K. Use of Ceftolozane/tazobactam for the Treatment of Multidrug-resistant Pseudomonas aeruginosa Pneumonia in a Pediatric Patient with Combined Immunodeficiency (CID): A Case Report from a Tertiary Hospital in Saudi Arabia. Antibiotics (Basel). 2019 May 27;8(2):67. DOI: 10.3390/antibiotics8020067
[160] Aitken SL, Kontoyiannis DP, DePombo AM, Bhatti MM, Tverdek FP, Gettys SC, Nicolau DP, Nunez CA. Use of Ceftolozane/Tazobactam in the Treatment of Multidrug-resistant Pseudomonas aeruginosa Bloodstream Infection in a Pediatric Leukemia Patient. Pediatr Infect Dis J. 2016 Sep;35(9):1040-2. DOI: 10.1097/INF.0000000000001228
[161] Martín-Cazaña M, Grau S, Epalza C, Brañas P, Flores M, Olmedilla M, Blázquez-Gamero D. Successful ceftolozane-tazobactam rescue therapy in a child with endocarditis caused by multidrug-resistant Pseudomonas aeruginosa. J Paediatr Child Health. 2019 Aug;55(8):985-7. DOI: 10.1111/jpc.14388
[162] Escolà-Vergé L, Pigrau C, Almirante B. Ceftolozane/tazobactam for the treatment of complicated intra-abdominal and urinary tract infections: current perspectives and place in therapy. Infect Drug Resist. 2019;12:1853-67. DOI: 10.2147/IDR.S180905
[163] Kollef MH, Nováček M, Kivistik Ü, Réa-Neto Á, Shime N, Martin-Loeches I, Timsit JF, Wunderink RG, Bruno CJ, Huntington JA, Lin G, Yu B, Butterton JR, Rhee EG. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2019 Dec;19(12):1299-1311. DOI: 10.1016/S1473-3099(19)30403-7
[164] Aguilar G, Ferriols R, Martínez-Castro S, Ezquer C, Pastor E, Carbonell JA, Alós M, Navarro D. Optimizing ceftolozane-tazobactam dosage in critically ill patients during continuous venovenous hemodiafiltration. Crit Care. 2019 Apr;23(1):145. DOI: 10.1186/s13054-019-2434-5
[165] Elizabeth Davis S, Ham J, Hucks J, Gould A, Foster R, Ann Justo J, Nicolau DP, Bookstaver PB. Use of continuous infusion ceftolozane-tazobactam with therapeutic drug monitoring in a patient with cystic fibrosis. Am J Health Syst Pharm. 2019 Apr;76(8):501-4. DOI: 10.1093/ajhp/zxz011
[166] Popejoy MW, Long J, Huntington JA. Analysis of patients with diabetes and complicated intra-abdominal infection or complicated urinary tract infection in phase 3 trials of ceftolozane/tazobactam. BMC Infect Dis. 2017 May;17(1):316. DOI: 10.1186/s12879-017-2414-9
[167] Solomkin J, Hershberger E, Miller B, Popejoy M, Friedland I, Steenbergen J, Yoon M, Collins S, Yuan G, Barie PS, Eckmann C. Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI). Clin Infect Dis. 2015 May;60(10):1462-71. DOI: 10.1093/cid/civ097
[168] Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet. 2015 May;385(9981):1949-56. DOI: 10.1016/S0140-6736(14)62220-0
[169] Massip C, Mathieu C, Gaudru C, Miaut V, Floch P, Oswald E, Segonds C, Guet-Revillet H. In vitro activity of seven β-lactams including ceftolozane/tazobactam and ceftazidime/avibactam against Burkholderia cepacia complex, Burkholderia gladioli and other non-fermentative Gram-negative bacilli isolated from cystic fibrosis patients. J Antimicrob Chemother. 2019 Feb;74(2):525-8. DOI: 10.1093/jac/dky423




