[Vom Zugang zur Reserve: Antibiotikaresistenz unter den Erregern zentralvenöser Katheterinfektionen im Hinblick auf das antimikrobielle Spektrum gemäß der AWaRe Klassifizierung der WHO]
Gargee Anand 1Rijhul Lahariya 1
Ketan Priyadarshi 1
Asim Sarfraz 1
1 All India Institute of Medical Sciences, Patna, Bihar, India
Zusammenfassung
Zielsetzung: ZVK assoziierte Blutstrominfektionen (CLABSI) sind nach wie vor eine der Hauptursachen für Morbidität und Mortalität bei schwerkranken Patienten. Die zunehmende antimikrobielle Resistenz (AMR) verschärft die Herausforderungen bei der Behandlung, weshalb die Untersuchung von Resistenzmustern der Erreger von entscheidender Bedeutung ist. In der Studie wird die Empfindlichkeit von CLABSI-assoziierten Erregern gegenüber Antibiotika anhand der AWaRe Klassifizierung der WHO analysiert, um Erkenntnisse für eine gezielte Behandlung zu gewinnen und Strategien zur Infektionskontrolle zu stärken.
Methode: In der Beobachtungsstudie (2021–2024) wurden Daten von Intensivstationen für Erwachsene und Kinder ausgewertet, um die Häufigkeit von CLABSI, die mikrobielle Ätiologie und die Entwicklung der Empfindlichkeit gegenüber Antibiotika zu untersuchen. Wir kategorisierten die antimikrobiellen Substanzen auf der Grundlage des AWaRe-Klassifizierungssystems der WHO und analysierten ihre Empfindlichkeit gegenüber Access-, Watch- und Reserve-Antibiotika. Die statistische Analyse wurde mit SPSS Version 22 durchgeführt.
Ergebnisse: Unter 5.398 Patientenakten wurden 101 Fälle von CLABSI bestätigt. Die vorherrschenden Erreger waren Klebsiella (K.) pneumoniae (27,7%), Acinetobacter spp. (19,8%) und Candida spp. (17,8%). Bei den wichtigsten Erregern wurde ein besorgniserregender Rückgang der Empfindlichkeit gegenüber antimikrobiellen Mitteln der Kategorien Access und Watch festgestellt. K. pneumoniae wies einen starken Rückgang der Empfindlichkeit gegenüber Mitteln der Kategorie „Access“ auf, von 27,8% im Jahr 2021 auf 16,7% im Jahr 2023. Umgekehrt behielten die antimikrobiellen Mittel der Reservekategorie ihre 100%ige Wirksamkeit über den gesamten Studienzeitraum. Acinetobacter spp. wiesen bis 2024 eine Resistenz sowohl gegen antimikrobielle Mittel der Access- als auch der Watch-Kategorie auf. Pseudomonas aeruginosa zeigte einen drastischen Rückgang der Empfindlichkeit für die Kategorie Watch von 44,5% im Jahr 2021 auf 0% im Jahr 2023, während die Mittel der Kategorie Reserve wirksam blieben. Die Ergebnisse unterstreichen die zunehmende Abhängigkeit von antimikrobiellen Mitteln der Reserve und die abnehmende Wirksamkeit der First line Mittel. Darüber hinaus beobachteten wir eine Fluktuation der CLABSI-Raten, wobei die Infektionsraten im Jahr 2024 nach der Einführung verbesserter Infektionskontrollverfahren deutlich zurückgingen.
Schlussfolgerung: Die Studie verdeutlicht die eskalierenden Resistenzmuster von CLABSI-Erregern mit einem besorgniserregenden Rückgang der antimikrobiellen Wirksamkeit der Kategorien Access und Watch. Der AWaRe-Rahmen erweist sich als wertvoll für die Identifizierung kritischer Resistenztrends und zeigt die Notwendigkeit eines gezielten antimikrobiellen Stewardships. Die Priorisierung von Access-Antibiotika als Erstlinientherapien, die sich an lokalen Resistenzdaten orientiert, kann die Wirksamkeit von Reserve-Wirkstoffen erhalten. Ein strategischer Fokus auf die AWaRe-Klassifizierung in Verbindung mit rigorosen Infektionskontroll- und Stewardship-Programmen ist unerlässlich, um die steigende AMR-Bedrohung zu bekämpfen und die Therapieergebnisse in der Intensivpflege zu optimieren.
Schlüsselwörter
ZVK-assoziierte Blutstrominfektion, CLABSI, Antibiotikaresistenz, WHO AWaRe, Intensivtherapiestation, Antibiotika Stewardship, Infektionskontrolle, Erreger angepasste Therapie, Healthcare-assoziierte Infektionen
Introduction
Global prevalence data from the World Health Organization (WHO) indicates that the risk of healthcare-associated infections (HAI) is particularly elevated in intensive care units (ICUs), affecting approximately 30% of ICU patients and resulting in significant morbidity and mortality [1]. The prevalence of HAIs differs markedly between developed and developing nations, with incidence rates of 7% and 10% respectively among hospitalized patients [1]. Among all HAIs, CLABSIs represent a substantial economic burden, with an estimated per-case cost of USD 46,000 [2]. A literature review indicates that CLABSIs significantly extend ICU length of stay, with reported excess hospitalization periods ranging from 2.7 to 48.5 days compared to non-infected patients [3]. CLABSIs not only carry substantial risks of illness and death but also demand more intensive and costly treatments compared to other HAIs, resulting in an exceptionally high burden on both patient care and hospital resources. The collective burden of CLABSIs has been estimated as equivalent to the eigth leading cause of death in the United States [4]. The emergence of antimicrobial resistance (AMR) coupled with biofilm formation on medical devices, particularly vascular catheters, presents significant therapeutic challenges [4]. The WHO developed the AWaRe (Access, Watch, Reserve) classification framework to address escalating AMR concerns while preserving therapeutic efficacy of critical antimicrobials [5]. This classification system strategically categorizes antimicrobial agents into three groups based on their therapeutic importance and resistance potential, and aims to mitigate the global health threat posed by AMR through enhanced surveillance, stewardship, and reduction of inappropriate antimicrobial consumption, strategically categorizing antimicrobials to optimize their use in healthcare settings [5], [6]. The present study presents an in-depth analysis of antimicrobial susceptibility profiles in CLABSI-associated pathogens, leveraging the WHO’s AWaRe classification system to offer novel insights into resistance patterns and inform targeted treatment strategies. Hence, the present study examined three key aspects of CLABSIs: incidence rates, microbial etiology, and antimicrobial susceptibility patterns, analyzed through the WHO’s AWaRe framework, to strengthen the synergy between infection prevention and antimicrobial stewardship programs.
Methods
Study design
This observational cross-sectional study encompassed patients from both adult and pediatric ICUs between 2021 and 2024. Inclusion criteria specified central line placement for more than 2 calendar days. Blood cultures were obtained for microbiological evaluation from patients presenting with clinical signs of bloodstream infection/sepsis. Cases of secondary bloodstream infections were excluded from the analysis. Standardized surveillance definitions of CLABSIs as per Centers for Disease Control and Prevention, National Healthcare Safety Network (CDC, NHSN) were followed [7]. An isolate was classified as multidrug-resistant (MDR) when it was non-susceptible to at least one antimicrobial agent in three or more antimicrobial classes [8]. The CLABSI rate was calculated as: (number of CLABSI/total central line days) ×1,000, expressed as CLABSI per 1,000 central line days [7]. Interpretive breakpoints for antimicrobial susceptibility testing established by CLSI (Clinical and Laboratory Standards Institute), M 100 guidelines for bacterial isolates were used [9]. Relevant data were collected and antimicrobial susceptibility patterns were analyzed using the WHO’s AWaRe classification [5].
Data collection
Patient data were retrospectively extracted from two institutional databases: the Hospital Information System (HIS) and HAI surveillance records. The HIS provided microbiological data, including blood culture results and antimicrobial susceptibility profile. HAI surveillance forms were used for gathering demographic information, clinical diagnosis, central line insertion sites, ICU length of stay, mortality/patient outcomes, and daily clinical assessments for catheter-related infection manifestations.
Patient and public involvement
In this study, there was no patient or public involvement, as the data were solely collected from the records department.
Statistical analysis
All relevant data were entered in a Microsoft Excel 2019 spreadsheet. Normality distribution for all continuous variables was tested using Q-Q plots, histograms, and the Shapiro-wilk test. Continuous variables were expressed using mean (±SD)/ median (IQR) according to their normality, while categorical variables were expressed as percentages/proportions. as appropriate. Bivariate comparison of categorical variables was performed using the Chi-squared test and Fisher’s exact test. Graphs depicting antimicrobial susceptibility trend as per WHO’s AWaRe classification were made using Microsoft Excel 2019. Statistical analysis was conducted using Statistical Package for Social Sciences (SPSS) version 22. A p-value of <0.05 was designated as statistically significant.
Results
Over the four-year (2021–2024) study period, records of 5398 patient who met the predefined inclusion criteria were assessed, of whom 101 patients developed CLABSI with Laboratory Confirmed Bloodstream Infection 1 (LCBI 1) criteria as per the CDC, NHSN surveillance criteria. The annual incidence of CLABSI is shown in Table 1 [Tab. 1], and overall incidence of CLABSI is depicted in Table 2 [Tab. 2] by type of ICU.
Table 1: Annual incidence of CLABSI (2021–2024) 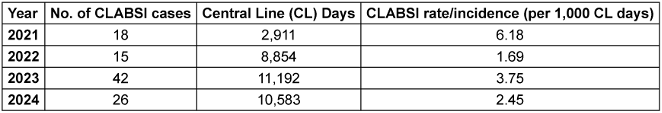
Table 2: ICU-specific incidence of CLABSI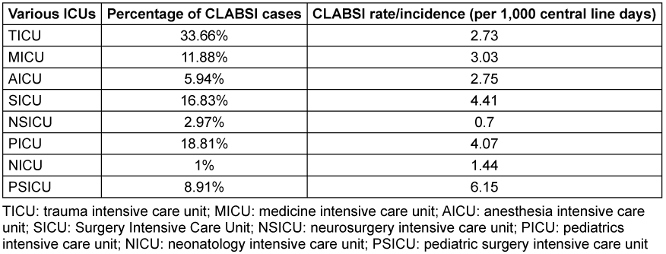
CLABSI more commonly occurred in patients having femoral access (22 CLABIS/150 femoral line). Analysis revealed a statistically significant predilection for CLABSIs among patients with femoral venous catheterization (p=0.001*).
Microbiological analysis of CLABSIs revealed a predominance of Gram-negative organisms (76/101; 75.3%), with Candida spp. (18/101; 17.8%) and Gram-positive organisms (7/101; 6.9%) comprising the remaining isolates (Table 3 [Tab. 3]).
Table 3: Microorganisms isolated from CLABSI cases (n=101)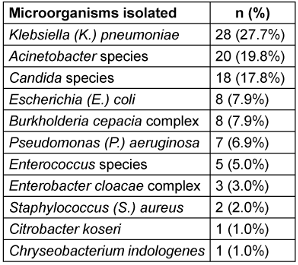
Analysis of antimicrobial susceptibility patterns across the WHO AWaRe categories revealed distinct temporal trends among isolated pathogens. K. pneumoniae exhibited a declining trend in Access-category susceptibility from 27.8% (2021) to 16.7% (2023), with a slight increase to 25.9% in 2024. Watch-category susceptibility showed a marked decrease from 37.5% (2021) to 2.5% (2022), followed by gradual increase to 15.3% (2024). Reserve-category antimicrobials maintained 100% efficacy throughout the study period. Acinetobacter spp. demonstrated fluctuating Access-category susceptibility: 22.2% (2022), increasing to 27.8% (2023), before declining to 0% (2024). Watch-category susceptibility showed consistently low rates, peaking at 9.52% (2023). Reserve antimicrobials maintained 100% efficacy from 2022–2024. E. coli susceptibility to Access-category antimicrobials decreased from 25% (2023) to 0% (2024), with a parallel decline in Watch-category susceptibility from 8.9% to 0%. However, Reserve-category antimicrobials maintained 100% efficacy. Burkholderia spp. maintained consistent Access-category susceptibility (100%) when isolated, while Watch-category susceptibility declined from 83.3% (2021) to 50% (2024). P. aeruginosa showed variable Watch-category susceptibility, decreasing from 44.5% (2021) to 0% (2023), with Reserve-category susceptibility declining from 66.7% (2021–2022) to 50% (2023).
Among Gram-positive organisms, Enterococcus spp. showed decreasing Access-category susceptibility from 50% (2021) to 0% (2023–2024), with Watch-category susceptibility declining from 50% (2021) to 33.3% (2023–2024). Reserve-category efficacy varied from 100% to 0%. S. aureus maintained relatively high Access-category susceptibility (60–80%), with Watch-category susceptibility increasing from 33.3% to 66.7%, and consistent Reserve-category efficacy at 100%. Overall susceptibility of access, Watch- and Reserve-category antimicrobials for various isolated microorganisms among CLABSI patients are shown in Figure 1 [Fig. 1]. Annual susceptibility trend (2021–2024) for various antimicrobials among isolated organisms is depicted in Table 4 [Tab. 4].
Table 4: Annual susceptibility trend (2021–2024) of various antibiotics against various CLABSI isolates (CLABSI cases 101)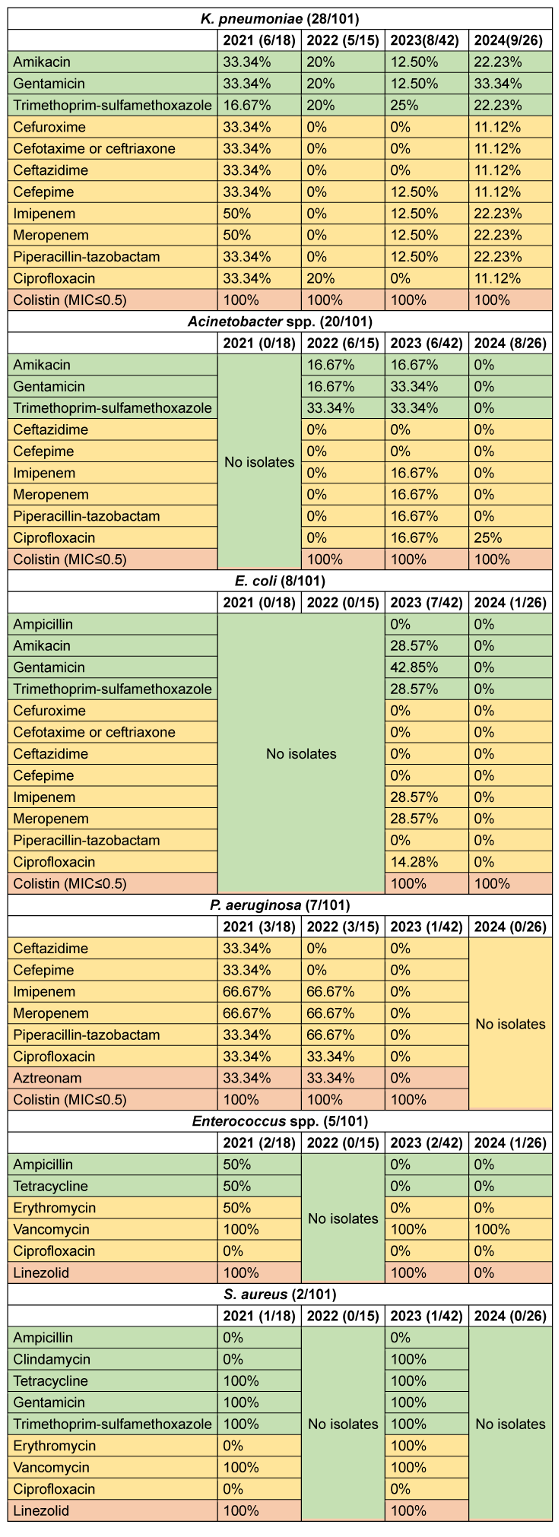
Figure 1: Susceptibility of Access, Watch and Reserve antibiotics among CLABSI isolates by year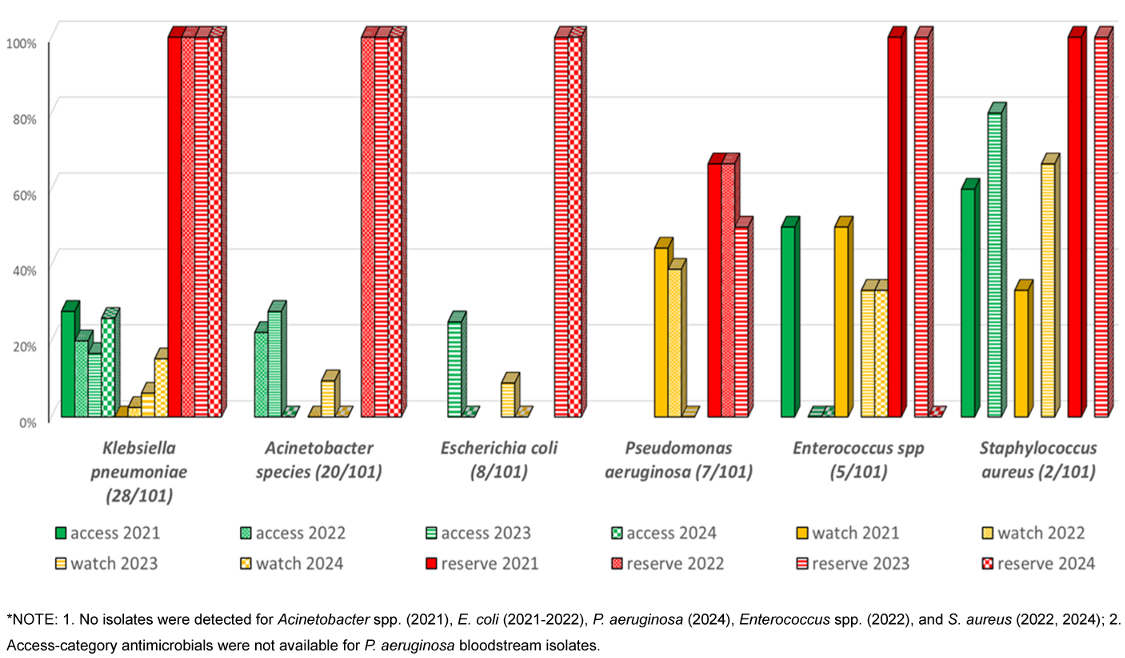
Discussion
This groundbreaking study is the first to analyse CLABSI pathogens’ AMR using the WHO’s AWaRe framework, offering critical insights for targeted treatment strategies. Analysis of CLABSI incidence over the four-year surveillance period (2021–2024) revealed notable variations. The baseline CLABSI rate in 2021 was 6.18 per 1,000 central line days, which demonstrated a substantial decline to 1.69 per 1,000 central line days in 2022, representing a 72.7% reduction. However, 2023 witnessed an increase to 3.75 per 1,000 central line days, followed by a subsequent decrease to 2.45 per 1,000 central line days in 2024.
This fluctuation in CLABSI rates warrants careful interpretation. The initial high rate in 2021 can be attributed to the lesser number of ICUs under surveillance and it might reflect the baseline period before implementation of enhanced prevention protocols. Following the elevated CLABSI rates in 2023 (3.75 per 1,000 central line days), implementation of enhanced insertion and maintenance-bundle practices led to a significant reduction in infection rates to 2.45 per 1,000 central line days in 2024, representing a 34.7% decrease. Notably, the central line utilization showed a progressive increase from 2,911 days in 2021 to 10,583 days in 2024, suggesting expanded critical care services or increased patient complexity. This increased device utilization might have contributed to the observed variations in infection rates.
These findings align with the published literature reporting CLABSI rates of 5 per 1,000 catheter days, while other Indian studies reported CLABSI rates ranging from 0.48 to 27 per 1,000 catheter days in various healthcare settings [10], [11]. The observed temporal variations underscore the dynamic nature of HAIs and emphasize the need for sustained vigilance in prevention strategies.
Statistical analysis showed a significantly greater predilection for CLABSI occurrence in the presence of femoral catheterization (p-value <0.001*) [12], [13]. The differential risk of CLABSIs across insertion sites can be attributed to anatomical variations, local microbiological colonization patterns, and site-specific mechanical factors.
The etiological spectrum of CLABSI revealed a predominance of Gram-negative organisms, constituting 75.2% of CLABSIs. Among these, K. pneumoniae emerged as the primary pathogen (27.7%), followed by Acinetobacter spp. (19.8%). This microbial distribution pattern corresponds with the literature, which documents the predominance of Gram-negative organisms in device-associated bloodstream infections [14].
Antimicrobial susceptibility testing of all CLABSI isolates revealed substantial AMR to first-line agents, a finding that aligns with a previous study [15].
The analysis of antimicrobial susceptibility patterns reveals concerning trends in pathogen resistance profiles across the WHO AWaRe classification framework. Analysis revealed worrying AMR patterns among predominant pathogens, with K. pneumoniae showing a progressive decline in Access-category susceptibility (27.8% to 16.7%) and Acinetobacter spp. demonstrating complete resistance to both Access- and Watch-categories by 2024. Notably, P. aeruginosa exhibited significant resistance development, with Watch-category susceptibility declining from 44.5% to 0% and Reserve-category efficacy decreasing from 66.7% to 50%. Despite these alarming trends, Access-category antimicrobials maintained better susceptibility profiles compared to Watch-category agents for most isolates. The sustained efficacy of Reserve-category antimicrobials (100% susceptibility) among major Gram-negative pathogens, while therapeutically promising, raises concerns about increasing reliance on last-resort antimicrobials. This pattern of escalating resistance to first-line agents, necessitating increased usage of Reserve antimicrobials, underscores the critical need for robust antimicrobial stewardship programs to preserve therapeutic options across all AWaRe categories, hence cascade reporting of antimicrobial susceptibility test (AST) results is of utmost importance.
Among Gram-positive organisms, the decreasing susceptibility of Enterococcus spp. to Access- and Watch-category antimicrobials, coupled with variable Reserve-category efficacy, suggests emerging resistance patterns requiring careful monitoring. Conversely, S. aureus maintained relatively favourable susceptibility profiles, particularly to Access-category agents, potentially reflecting effective infection control measures.
A paradigm shift is necessary in prescribing practices within ICUs, emphasizing pathogen-directed therapy guided by local susceptibility data rather than defaulting to broad-spectrum Watch- and Reserve-group antimicrobials. Prioritizing Access antimicrobials as first-line therapies, wherever appropriate, will help preserve efficacy of Watch and Reserve agents [16]. The most urgent step needed now is to implement targeted bundle care practices, antimicrobial stewardship strategies aligned with the WHO’s AWaRe classification, even in critical care settings [17], [18].
Conclusions
The study highlights a precarious situation where the efficacy of Access/Watch antimicrobials is compromised and thus increased reliance is placed on Reserve antimicrobials. This complicates patient management and poses a global health threat by AMR. Thus, prioritizing Access antimicrobials as first-line, where appropriate, will preserve the efficacy of Watch and Reserve agents, mitigating the emergence of extensively drug-resistant strains. This strategy, coupled with
- implementation of pathogen-directed therapy based on local resistance data rather than empiric broad-spectrum antimicrobial use,
- development of targeted antimicrobial stewardship programs aligned with the WHO AWaRe framework, specifically adapted for critical care settingsrigorous infection, and
- control measures and continuous surveillance
offers a promising path to combat AMR in HAIs while adhering to the WHO’s AWaRe even in critical care settings.
Notes
Competing interests
The authors declare that they have no competing interests.
Funding sources
The authors hereby declare that no financial support was received for this study.
Authors’ ORCIDs
- Anand G: https://orcid.org/0009-0008-0473-389X
- Lahariya R: https://orcid.org/0009-0003-5769-4509
- Priyadarshi K: https://orcid.org/0000-0003-4623-3523
- Sarfraz A: https://orcid.org/0000-0002-6256-7649
References
[1] World Health Organization. Report on the burden of endemic health care-associated infection worldwide.World Health Organization; 2011. p. 40. Available from: https://iris.who.int/handle/10665/80135[2] Haddadin Y, Annamaraju P, Regunath H. Central Line-Associated Blood Stream Infections, 2022 Nov 26. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025 Jan.
[3] Barnett AG, Graves N, Rosenthal VD, Salomao R, Rangel-Frausto MS. Excess length of stay due to central line-associated bloodstream infection in intensive care units in Argentina, Brazil, and Mexico. Infect Control Hosp Epidemiol. 2010 Nov;31(11):1106-14. DOI: 10.1086/656593
[4] Wenzel RP. Health care-associated infections: major issues in the early years of the 21st century. Clin Infect Dis. 2007 Jul;45(Suppl 1):S85-8. DOI: 10.1086/518136
[5] The WHO AWaRe (Access, Watch, Reserve) antibiotic book. Geneva: World Health Organization; 2022. p. 697.
[6] Zanichelli V, Sharland M, Cappello B, Moja L, Getahun H, et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull WHO. 2023 Feb 10;101(4):290-6. DOI: 10.2471/BLT.22.288614
[7] National Healthcare Safety Network (NHSN). Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection). Atlanta, GA: CDC; 2025 Jan [cited 2025 Jan 24]. p. 46. Available from: https://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf?bcsi-ac-e0643eac7b939e3d=2325FF7500000002JFYeQSLbjK8VAWSm4pmXbJRbUICrHQAAAgAAAFRsbwCEAwAAPwAAAB3SCAA=
[8] Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268-81. DOI: 10.1111/j.1469-0691.2011.03570.x
[9] CLSI. M 100 – Performance Standards for Antimicrobial Susceptibility Testing. 31th ed. Clinical and Laboratory Standards Institute; 2021 Mar.
[10] Singhal T, Shah S, Thakkar P, Naik R. The incidence, aetiology and antimicrobial susceptibility of central line-associated bloodstream infections in intensive care unit patients at a private tertiary care hospital in Mumbai, India. Indian J Med Microbiol. 2019;37(4):521-6. DOI: 10.4103/ijmm.IJMM_20_3
[11] Gopal PB. The Clasp of CLABSI. Indian J Crit Care Med. 2020 Jan;24(1):3-5. DOI: 10.5005/jp-journals-10071-23335
[12] Lee KH, Cho NH, Jeong SJ, Kim MN, Han SH, Song YG. Effect of Central Line Bundle Compliance on Central Line-Associated Bloodstream Infections. Yonsei Med J. 2018 May;59(3):376-82. DOI: 10.3349/ymj.2018.59.3.376
[13] Hassan N, Hammodi A, Alhubail R, Rayyan N. Effect of insertion site on risk of central line associated blood stream infection in critically ill patients. Ann Vasc Med Res. 2017 Jan 18;4(7):1081.
[14] Darji SM, Patel N. Central line associated blood stream infection: microbiological profile and its antimicrobial susceptibility pattern at tertiary care centre. J Pure Appl Microbiol. 2023 Apr 21;17(2):911-8. DOI: 10.22207/JPAM.17.2.18.
[15] Amanati A, Sajedianfard S, Khajeh S, Ghasempour S, Mehrangiz S, Nematolahi S, Shahhosein Z. Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect Dis. 2021 Jul;21(1):636. DOI: 10.1186/s12879-021-06243-z
[16] Mandal P, Asad M, Kayal A, Biswas M. Assessment of use of World Health Organization access, watch, reserve antibiotics and core prescribing indicators in pediatric outpatients in a tertiary care teaching hospital in Eastern India. Perspect Clin Res. 2023;14(2):61-7. DOI: 10.4103/picr.picr_22_22
[17] Funiciello E, Lorenzetti G, Cook A, Goelen J, Moore CE, Campbell SM, Godman B, Tong D, Huttner B, Chuki P, Sharland M. Identifying AWaRe indicators for appropriate antibiotic use: a narrative review. J Antimicrob Chemother. 2024 Dec;79(12):3063-77. DOI: 10.1093/jac/dkae370
[18] Singh S, Sharma A, Dhawan M, Sharma SP. Assessment of the Level of Awareness and Degree of Implementation of Central Line Bundles for Prevention of Central Line-associated Blood Stream Infection: A Questionnaire-based Observational Study. Indian J Crit Care Med. 2024 Sep;28(9):847-53. DOI: 10.5005/jp-journals-10071-24785




